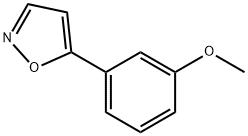
5-(3-Methoxyphenyl)isoxazole, 95% synthesis
- Product Name:5-(3-Methoxyphenyl)isoxazole, 95%
- CAS Number:1194374-23-6
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
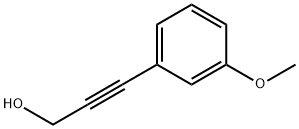
27913-19-5
26 suppliers
$165.00/1g
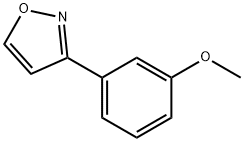
1093649-61-6
0 suppliers
inquiry

1194374-23-6
12 suppliers
$24.39/1g
Yield:1194374-23-6 44% ,1093649-61-6 41%
Reaction Conditions:
with nickel(II) triflate;N-iodo-succinimide;N-(9-fluorenylmethoxycarbonyloxy)hydroxylamine in toluene at 111; for 5 h;Sealed tube;
Steps:
9 Example 9Method for preparing isoxazole derivative, two kinds of isoxazole derivative obtained,Isoxazole derivative I is 5- (3-methoxyphenyl) isoxazole, and isoxazole derivative II is 3- (3-methoxyphenyl) isoxazole;
The preparation process is: adding propargyl alcohol derivative, halogen source and acid in a sealed tube,The reaction was carried out under heating and reflux at 111 ° C, and hydroxylamine was added after the disappearance of the propargyl alcohol derivative was monitored by TLC.After 5 hours of reaction, saturated brine was added to quench the reaction. The organic phase was extracted with ethyl acetate and dried over anhydrous sodium sulfate.Concentration yields the isoxazole derivative, which can be purified by column chromatography to calculate the yield:The yield of isoxazole derivative I was 44%, the yield of isoxazole derivative II was 41%, and the total yield of isoxazole derivative was 85%.Among them, propargyl alcohol, halogen source, hydroxylamine,The solvent and acid loading was 3- (3-methoxyphenyl) prop-2-yn-1-ol (2 mmol).NIS (2 mmol), N- (9-fluorenylmethoxycarbonyl) hydroxylamine (2.1 mmol), toluene (15 mL) and nickel triflate (0.45 mmol).
References:
CN110483430,2019,A Location in patent:Paragraph 0096-0101
58316-11-3
14 suppliers
$75.00/1g

1194374-23-6
12 suppliers
$24.39/1g
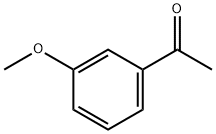
586-37-8
326 suppliers
$10.00/5g

1194374-23-6
12 suppliers
$24.39/1g