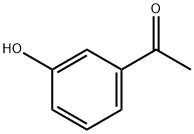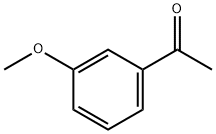
3-Methoxyacetophenone synthesis
- Product Name:3-Methoxyacetophenone
- CAS Number:586-37-8
- Molecular formula:C9H10O2
- Molecular Weight:150.17
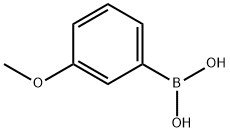
10365-98-7
417 suppliers
$9.00/10g
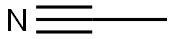
75-05-8
1044 suppliers
$10.00/10g

586-37-8
322 suppliers
$10.00/5g
Yield:586-37-8 84%
Reaction Conditions:
with 1,10-Phenanthroline;nickel(II) bromide 2-methoxyethyl ether complex;sodium hydrogencarbonate in water at 100; for 5 h;Autoclave;
Steps:
General procedure for the synthesis of arylketones 3a-y:
General procedure: The reaction was carried out in an autoclave containing a 10 mL Teflon reactiontube. NiBr2·diglyme (5 mol%), 1,10-phen (10 mol%) and a magnetic stir bar wereplaced in the tube. Then, arylboronic acid (1.0 mmol), NaHCO3 (2.0 equiv), H2O (2.0mmol) and alkyl nitrile (1.0 mL) were added to the tube. After that the autoclave wascapped with a stopper. The autoclave was cool down by liquid nitrogen, then createdvacuum at this temperature and added HCFC-244bb (2.0 mL, 2.6 g) by self-suction.Finally the autoclave was wormed in an oil bath at 100 °C for 5 h. After the reaction,the autoclave was cooled to room temperature and vented the excess HCFC-244bb carefully. Water (30 mL) was added to the mixture, and the mixture was extractedwith dichloromethane (3 x 15 mL). The organic layers were washed with brine, driedover Na2SO4, and evaporated the organic solvent by rotatory evaporator. The crudeproduct was then purified by column chromatography.
References:
Tu, Dong-Huai;Li, Yang;Zhao, Bo;Gu, Yu-Jie;Wang, Bo;Lu, Ju-You;Lu, Jian [Synlett,2018,vol. 29,# 5,art. no. ST-2017-W0661-L,p. 593 - 596] Location in patent:supporting information
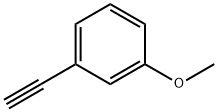
768-70-7
144 suppliers
$12.00/250mg

586-37-8
322 suppliers
$10.00/5g
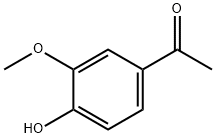
498-02-2
482 suppliers
$5.00/1g

586-37-8
322 suppliers
$10.00/5g
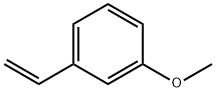
626-20-0
62 suppliers
$97.00/1g

586-37-8
322 suppliers
$10.00/5g