
5-(4-METHYLPHENYL)-1H-PYRAZOLE-3-CARBOXYLIC ACID synthesis
- Product Name:5-(4-METHYLPHENYL)-1H-PYRAZOLE-3-CARBOXYLIC ACID
- CAS Number:46413-67-6
- Molecular formula:C11H10N2O2
- Molecular Weight:202.21

55558-81-1
0 suppliers
inquiry

46413-67-6
32 suppliers
$23.00/1g
Yield:46413-67-6 87%
Reaction Conditions:
with hydrazine hydrate;acetic acid at 20;
Steps:
1 4.1.1.1. 5-(4-Methylphenyl)-1H-pyrazole-3-carboxylic acid (1)
4.1.1.1
5-(4-Methylphenyl)-1H-pyrazole-3-carboxylic acid (1)
0.4 g of 4-(4-methylphenyl)-2,4-dioxobutanoic acid was dissolved in 20 ml of glacial acetic acid in 50 ml round-bottom flask.
In a stirred solution, 280 μL (3 eq.) of hydrazine monohydrate (N2H4·H2O) was added dropwise.
The color of solution was changed to pale yellow immediately after addition of first amount of hydrazine.
The progress of reaction was monitored by TLC. After completion, reaction mixture was poured in 50 ml H2O and left to stir overnight.
White precipitate was collected, dried on air and recrystallized from the mixture of hexane/EtOH (~9:1), giving 0.34 g of product (87% of theoretical yield). 4.1.1.1
5-(4-Methylphenyl)-1H-pyrazole-3-carboxylic acid (1)
0.4 g of 4-(4-methylphenyl)-2,4-dioxobutanoic acid was dissolved in 20 ml of glacial acetic acid in 50 ml round-bottom flask.
In a stirred solution, 280 μL (3 eq.) of hydrazine monohydrate (N2H4·H2O) was added dropwise.
The color of solution was changed to pale yellow immediately after addition of first amount of hydrazine.
The progress of reaction was monitored by TLC. After completion, reaction mixture was poured in 50 ml H2O and left to stir overnight.
White precipitate was collected, dried on air and recrystallized from the mixture of hexane/EtOH (~9:1), giving 0.34 g of product (87% of theoretical yield).4.1.2.1
5-(4-Methylphenyl)-1H-pyrazole-3-carboxylic acid (1)
White solid; mp = 253-254 °C (hexane/EtOH); C11H10N2O2, Mw = 202.21; ESI-MS: Calculated for C11H11N2O2 [M+H]+: 203.08150, measured: 203.08063; IR (ν, cm-1): 3264 (N-H), 1696 (C=O), 1500, 1198; 1H NMR (500 MHz, DMSO-d6) δ(ppm): 2.30 (s, 3 H), 7.14 (s, 1 H), 7.22 (d, J = 7.83 Hz, 2 H), 7.71 (d, J = 8.31 Hz, 2 H), 13.38 (br).
13C NMR (125 MHz, DMSO-d6) δ(ppm): 20.85, 104.91, 125.25, 128.13, 129.48, 137.58, 140.09, 147.44, 162.04.
References:
Cvijeti?, Ilija N.;Tan?, Muhammet;Jurani?, Ivan O.;Verbi?, Tatjana ?.;Supuran, Claudiu T.;Drakuli?, Branko J. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 15,p. 4649 - 4659]
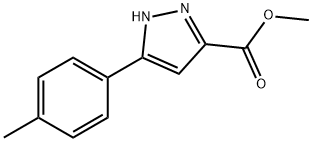
192701-73-8
39 suppliers
$17.00/100mg

46413-67-6
32 suppliers
$23.00/1g
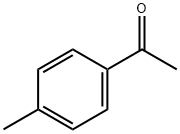
122-00-9
713 suppliers
$5.00/25g

46413-67-6
32 suppliers
$23.00/1g

127404-74-4
5 suppliers
inquiry

46413-67-6
32 suppliers
$23.00/1g
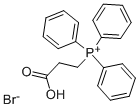
51114-94-4
192 suppliers
$16.80/5G

46413-67-6
32 suppliers
$23.00/1g