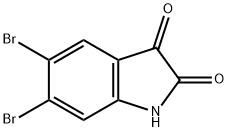
5,6-Dibromo-1H-indole-2,3-dione synthesis
- Product Name:5,6-Dibromo-1H-indole-2,3-dione
- CAS Number:17826-05-0
- Molecular formula:C8H3Br2NO2
- Molecular Weight:304.92
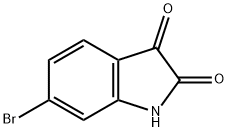
6326-79-0

17826-05-0
General procedure for the synthesis of 5,6-dibromoindoline-2,3-dione from 6-bromodihydroindole-2,3-dione: Bromine (0.88 mmol) was slowly added to a solution of acetic acid (9 mL) containing 6-bromoindigo red (0.88 mmol). The reaction mixture was stirred under reflux conditions for 48 hours. Upon completion of the reaction, the mixture was cooled to 0 °C, the solid product was collected by filtration and washed with acetic acid. Subsequently, the crude product was dried in an oven. Purification of the crude product by recrystallization from acetic acid afforded an orange-colored pure product in 57% yield; melting point >270 °C (decomposition). The structure of the product was confirmed by nuclear magnetic resonance hydrogen (1H NMR, 300 MHz, DMSO-d6) and carbon (13C NMR, 75 MHz, DMSO-d6) spectra: 1H NMR (DMSO-d6) δ 7.23 (1H, s, H-7), 7.82 (1H, s, H-4), 11.22 (1H, brs, NH); 13C NMR (DMSO-d6) δ 117.40, 117.64, 119.61, 129.33, 133.81, 150.67, 159.83, 183.12.

6326-79-0
276 suppliers
$6.00/1g

17826-05-0
43 suppliers
$105.00/100mg
Yield:17826-05-0 76%
Reaction Conditions:
with NBS in N,N-dimethyl-formamide at 25; for 12 h;Inert atmosphere;
Steps:
4.1.2. General Procedure II: Synthesis of Multi-Substituted Isatin Derivatives (4a-4c)
General procedure: To a solution of compound 3 (3.0 mmol) in N, N-dimethylformamide (2.5 mL) wasadded NBS (3.3 mmol). The resulting reaction mixture was stirred at 25 C for 12 h. Afterthe reaction finished, the mixture was diluted with water (10 mL), the resultant precipitatefiltered, and the precipitate was dried in vacuo. The crude product was chromatographedgradiently on silica gel with PE/EA (10:14:1) to give the compounds 4a-4c.
References:
Ding, Ying;Zhao, Lianbo;Fu, Ying;Hao, Lei;Fu, Yupeng;Yuan, Yuan;Yu, Peng;Teng, Yuou [Molecules,2021,vol. 26,# 1,art. no. 176]
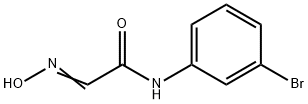
65971-74-6
5 suppliers
inquiry

17826-05-0
43 suppliers
$105.00/100mg
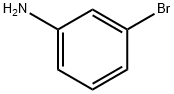
591-19-5
334 suppliers
$10.00/10g

17826-05-0
43 suppliers
$105.00/100mg
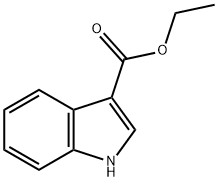
776-41-0
165 suppliers
$33.00/1g

17826-05-0
43 suppliers
$105.00/100mg
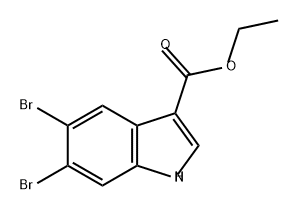
857809-62-2
0 suppliers
inquiry

17826-05-0
43 suppliers
$105.00/100mg