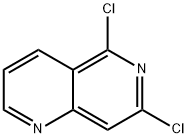
5,7-dichloro-1,6-naphthyridine synthesis
- Product Name:5,7-dichloro-1,6-naphthyridine
- CAS Number:337958-60-8
- Molecular formula:C8H4Cl2N2
- Molecular Weight:199.04
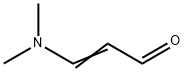
927-63-9
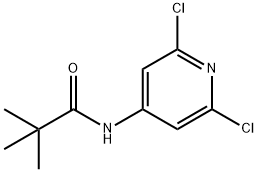
1345456-45-2

337958-60-8
The general procedure for the synthesis of 5,7-dichloro-1,6-naphthyridines from 3-dimethylaminoacrolein and N-(2,6-dichloropyridin-4-yl)pivalamide is as follows: Intermediate 7: Synthesis of 5,7-dichloro-1,6-diazanaphthalene 1. dissolve N-(2,6-dichloro-4-pyridinyl)-2,2-dimethylpropanamide (1 g, 4.05 mmol) in tetrahydrofuran (THF, 10 ml) and cool to below -60 °C under nitrogen protection. 2. Slowly add n-butyllithium (4.05 ml, 10.12 mmol, 2.5 M hexane solution), keep the temperature below -60°C, and control the addition time within 30 minutes. After addition, continue stirring below -70°C for 1 hour. 3. THF (2 ml) solution of (2E)-3-(dimethylamino)-2-propenal (0.607 ml, 6.07 mmol) was added dropwise over 30 min, keeping the reaction temperature below -60 °C. After dropwise addition, the reaction mixture was stirred below -70°C for 20 min and then slowly warmed up to room temperature. 4. The progress of the reaction was monitored by LCMS and after confirming complete consumption of the feedstock, the reaction was quenched with 5 M hydrochloric acid (5 ml) and the mixture was refluxed overnight. 5. After LCMS showed good conversion of the product, the reaction mixture was cooled to room temperature, alkalized with solid potassium carbonate (K2CO3) and subsequently extracted with ethyl acetate (EtOAc, 4 x 25 ml). 6. The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated to give a brown solid crude product. 7. The crude product was purified by column chromatography using a 40+M silica gel column, eluting first with 12% ether in cyclohexane solution for 2 column volumes (CV), then with a gradient of 12%-63% ether in cyclohexane solution for 10 CV, and finally keeping the elution in 63% ether in cyclohexane solution until completion. 8. The fractions containing the target product were collected, combined and the solvents evaporated to afford 5,7-dichloro-1,6-naphthyridine as a yellow solid (0.42 g). LCMS (Method B): retention time (Rt) = 0.89 min, molecular ion peak (MH+) = 199/201.
2587-02-2
313 suppliers
$12.40/250mg

337958-60-8
185 suppliers
$26.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: triethylamine / dichloromethane / Cooling with ice
2.1: n-butyllithium / tetrahydrofuran; hexanes / 1.5 h / -70 - -60 °C / Inert atmosphere
2.2: -70 - 20 °C
References:
GLAXO GROUP LIMITED;ATKINSON, Francis Louis;BARKER, Michael David;DOUAULT, Clement;GARTON, Neil Stuart;LIDDLE, John;PATEL, Vipulkumar Kantibhai;PRESTON, Alexander George Steven;WILSON, David Matthew WO2011/134971, 2011, A1
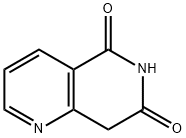
1345461-33-7
14 suppliers
inquiry

337958-60-8
185 suppliers
$26.00/250mg
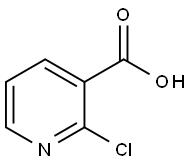
2942-59-8
823 suppliers
$5.00/25g

337958-60-8
185 suppliers
$26.00/250mg