
5-ACETOXYMETHYL-2-FURANCARBOXYLIC ACID synthesis
- Product Name:5-ACETOXYMETHYL-2-FURANCARBOXYLIC ACID
- CAS Number:90345-66-7
- Molecular formula:C8H8O5
- Molecular Weight:184.15
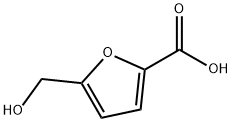
6338-41-6
212 suppliers
$5.00/100mg

108-24-7
0 suppliers
$14.00/250ML

90345-66-7
36 suppliers
$57.50/500mg
Yield:90345-66-7 93%
Reaction Conditions:
with triethylamine in diethyl ether at 20; for 14 h;Cooling with ice;
Steps:
2 Preparation Example 2 Preparation of 5-(acetoxymethyl)-2-furoic acid
15.64 g of HMFCA (104 mmol), 300 mL of ethyl ether, and 20.24 g of triethylamine (NEt3, 0.2 mol) were added into a twin neck bottle (250 mL) and stirred to be completely dissolved. 11.78 mL of acetic anhydride (124.8 mmol) was then slowly added into the twin neck bottle in an ice bath, and the ice bath was then removed after the addition of acetic anhydride for slowly warming up the reaction to room temperature. The reaction was continued at room temperature for 14 hours, and 3M HCl was then added into the twin neck bottle to acidify the solution in the twin neck bottle. The acidified solution was extracted by de-ionized water three times, and the organic phase of the extractions was collected and then dried by anhydrous MgSO4. The organic phase was concentrated to obtain a yellow solid. The yellow solid was washed by n-hexane and then dried to obtain 17.84 g of 5-(acetoxymethyl)-2-furoic acid product (yield=93%), as shown in Formula 1 with R1 being methyl group. The above reaction is shown in Formula 9. The product of Formula 9 had NMR spectra as below: 1H NMR (400 M Hz, CDCl3): 7.23 (d, 1H, J=3.5 Hz), 6.51 (d, 1H, J=3.5 Hz), 5.07 (s, 2H), 2.07 (s, 3H). 13C NMR (100 M Hz, CDCl3): 170.6, 162.5, 154.4, 144.0, 120.5, 112.5, 57.8, 20.7. The product of Formula 9 had mass spectrum as below: HRMS (EI, m/z): calcd. for C8H8O5 184.15. found 184.11 (M+).
References:
US9321744,2016,B1 Location in patent:Page/Page column 5; 6
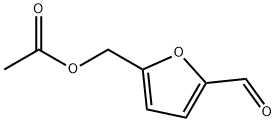
10551-58-3
152 suppliers
$22.00/1g
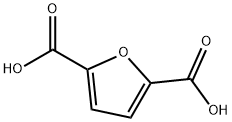
3238-40-2
475 suppliers
$6.00/1g

90345-66-7
36 suppliers
$57.50/500mg

10551-58-3
152 suppliers
$22.00/1g

90345-66-7
36 suppliers
$57.50/500mg
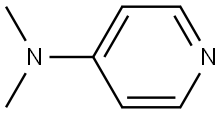
1122-58-3
1119 suppliers
$6.00/1g

6338-41-6
212 suppliers
$5.00/100mg

90345-66-7
36 suppliers
$57.50/500mg
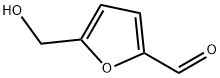
67-47-0
613 suppliers
$5.00/100mg

90345-66-7
36 suppliers
$57.50/500mg