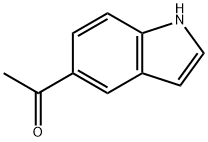
5-Acetylindole synthesis
- Product Name:5-Acetylindole
- CAS Number:53330-94-2
- Molecular formula:C10H9NO
- Molecular Weight:159.18
1670-81-1
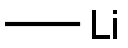
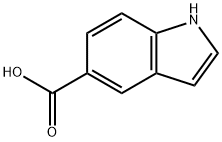
917-54-4

53330-94-2
Methyl lithium (1.6 M ether solution, 30 mL, 51.2 mmol) was added slowly and dropwise to a solution of anhydrous tetrahydrofuran (30 mL) of indole-5-carboxylic acid (2.5 g, 15.5 mmol) at 0 °C and under nitrogen protection. The reaction mixture was stirred at room temperature for 4 hours before the reaction was quenched with aqueous ammonium chloride solution and extracted with dichloromethane (3 x 50 mL). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 5-acetylindole (1.8 g, white solid).

1670-81-1
338 suppliers
$6.00/250mg
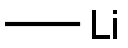
917-54-4
182 suppliers
$44.39/50ml

53330-94-2
121 suppliers
$27.00/100mg
Yield: 87.97%
Reaction Conditions:
in tetrahydrofuran at 0; for 12 h;Inert atmosphere;
Steps:
4.1.15. The synthesis of intermediate 29
The 1H-indole-5-carboxylic acid (1.5 g, 9.31 mmol) was dissolvedin THF under Ar atmosphere and CH3Li (1.5 M, 20.48 mL,30.71 mmol) was added dropwise at 0 °C. After stirring for 12 h, thereactionwas quenched with water, extracted with EA (3 x 150 mL), and the combined organic layers were then washed with brine,dried over anhydrous Na2SO4, and concentrated in vacuo to providethe crude product, which was purified by column chromatographywith petroleum/ethyl acetate (4: 1) to give 29 (1.30 g, 87.97%) as ayellow oil. The spectra data were consistent with the literature [29].
References:
Shuai, Wen;Li, Xinnan;Li, Wenlong;Xu, Feijie;Lu, Lixue;Yao, Hong;Yang, Limei;Zhu, Huajian;Xu, Shengtao;Zhu, Zheying;Xu, Jinyi [European Journal of Medicinal Chemistry,2020,vol. 197,art. no. 112308]
16078-34-5
79 suppliers
$27.00/250mg

53330-94-2
121 suppliers
$27.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

53330-94-2
121 suppliers
$27.00/100mg
889108-48-9
27 suppliers
inquiry

53330-94-2
121 suppliers
$27.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

53330-94-2
121 suppliers
$27.00/100mg