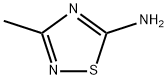
5-AMINO-3-METHYL-1,2,4-THIADIAZOLE synthesis
- Product Name:5-AMINO-3-METHYL-1,2,4-THIADIAZOLE
- CAS Number:17467-35-5
- Molecular formula:C3H5N3S
- Molecular Weight:115.16
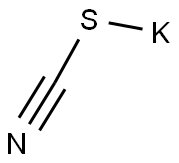
333-20-0
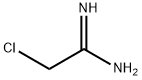
20846-52-0

17467-35-5
The general procedure for the synthesis of 5-amino-3-methyl-1,2,4-thiadiazole from potassium thiocyanate and chloroacetamidine was as follows: chloroacetamidine (5.1 g, 55 mmol) was dissolved in methanol (250 mL) and the resulting solution was cooled to 0 °C. Subsequently, potassium thiocyanate (5.3 g, 55 mmol) was added to the solution. The reaction mixture was stirred at room temperature for 12 hours. Upon completion of the reaction, the solution was concentrated under reduced pressure, diluted with ethyl acetate (200 mL) and insoluble solids were removed by filtration under reduced pressure. The filtrate was further concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography (silica gel, n-hexane:ethyl acetate = 4:1) to afford finally 5-amino-3-methyl-1,2,4-thiadiazole (2 g, 32% yield). The structure of the product was confirmed by 1H NMR (600 MHz, CD3OD), δ 3.27 (s, 3H).

333-20-0
464 suppliers
$13.50/100G

20846-52-0
36 suppliers
inquiry

17467-35-5
162 suppliers
$6.00/1g
Yield:17467-35-5 32%
Reaction Conditions:
in methanol at 0 - 20; for 12 h;
Steps:
40.40-4
Compound LXII (5.1 g, 55 mmol) was dissolved in methanol (250 mL) and the resulting solution was cooled to 0° C. Potassium thiocyanate (5.3 g, 55 mmol) was then added. The resulting solution was stirred for 12 hours at room temperature, concentrated under reduced pressure, diluted with ethyl acetate (200 mL), and filtered under reduced pressure to remove a solid. The filtrate was concentrated under reduced pressure, creating a solid which was filtered under reduced pressure to yield Compound LXIII (2 g (32%)). The filtrate was further concentrated and applied to column chromatography (SiO2, n-hex:EA=4:1) to yield Compound LXIII (2 g (32%)).1H NMR (600 MHz, CD3OD) δ 3.27 (s, 3H)
References:
US2012/264727,2012,A1 Location in patent:Page/Page column 53

130974-24-2
0 suppliers
inquiry

17467-35-5
162 suppliers
$6.00/1g
1141922-26-0
4 suppliers
inquiry

17467-35-5
162 suppliers
$6.00/1g
124-42-5
372 suppliers
$6.00/25g

333-20-0
464 suppliers
$13.50/100G

17467-35-5
162 suppliers
$6.00/1g
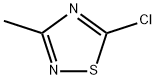
21734-85-0
91 suppliers
$26.00/100mg

17467-35-5
162 suppliers
$6.00/1g