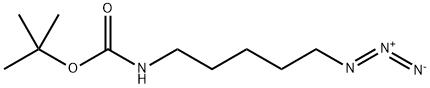
(5-Azidopentyl)carbaMic acid tert-butyl ester synthesis
- Product Name:(5-Azidopentyl)carbaMic acid tert-butyl ester
- CAS Number:129392-86-5
- Molecular formula:C10H20N4O2
- Molecular Weight:228.3
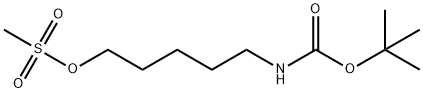
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg

129392-86-5
16 suppliers
inquiry
Yield:129392-86-5 86%
Reaction Conditions:
with sodium azide in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
tert-butyl (5-azidopentyl)carbamate (13-4).
13-3 (1.39 g, 3.88 mmol) and NaN3 (303.5 mg, 4.7 mmol) were dissolved in DMF (39 mL), which was stirred for 12 h at room temperature. The reaction mixture was diluted in EtOAc, washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was subjected to flash column chromatography (SiO2, CHCl3) to obtain 13-4 (764.7 mg, 86%). 1H NMR (500 MHz, CDCl3) δ 4.54 (broad s, 1H), 3.26 (t, J = 7.0 Hz, 2H), 3.11 (broad q, J = 6.5 Hz, 2H), 1.63-1.58 (m, 2H), 1.53-1.47 (m, 2H), 1.43 (s, 9H), 1.41-1.35 (m, 2H); HRMS (ESI, positive) m/z 251.1442 [M+Na]+ (calcd for C10H20N4O2 251.1478).
References:
Otsuki, Satsuki;Nishimura, Shinichi;Takabatake, Hisae;Nakajima, Kozue;Takasu, Yasuaki;Yagura, Toru;Sakai, Yuki;Hattori, Akira;Kakeya, Hideaki [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 6,p. 1608 - 1611] Location in patent:supporting information
75178-90-4
123 suppliers
$8.00/250mg

129392-86-5
16 suppliers
inquiry
142342-55-0
23 suppliers
$50.00/250mg

129392-86-5
16 suppliers
inquiry

2508-29-4
202 suppliers
$11.00/5g

129392-86-5
16 suppliers
inquiry
51644-96-3
167 suppliers
$23.00/1g

129392-86-5
16 suppliers
inquiry