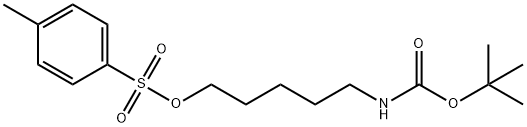
TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE synthesis
- Product Name:TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE
- CAS Number:118811-34-0
- Molecular formula:C17H27NO5S
- Molecular Weight:357.46
75178-90-4
132 suppliers
$8.00/250mg

98-59-9
626 suppliers
$9.00/5g
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg
Yield:118811-34-0 91%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 0 - 20; for 4.5 h;
Steps:
5-((tert-butoxycarbonyl)amino)pentyl 4-methylbenzenesulfonate (13-3).
To a stirred solution of 13-2 (1.97 g, 9.7 mmol) in CH2Cl2 (32 mL) on ice was added TsCl (2.78 g, 14.6 mmol), Et3N (4.0 mL, 28.9 mmol) and DMAP (117.4 mg, 0.96 mmol). After being stirred for 4.5 h, the reaction mixture was diluted with CHCl3, washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was subjected to flash column chromatography (SiO2, n-hexane/EtOAc = 1:0, 8:1, and then 4:1) to obtain 13-3 (3.14 g, 91%). 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 4.51 (broad s, 1H), 4.00 (t, J = 6.5 Hz, 2H), 3.06-3.02 (broad q, J = 6.5 Hz, 2H), 2.44 (s, 3H), 1.67-1.61 (m, 2H), 1.42-1.38 (m, 11H), 1.35-1.29 (m, 2H); HRMS (ESI, positive) m/z 380.1497 [M+Na]+ (calcd for C17H27NO5S 380.1502).
References:
Otsuki, Satsuki;Nishimura, Shinichi;Takabatake, Hisae;Nakajima, Kozue;Takasu, Yasuaki;Yagura, Toru;Sakai, Yuki;Hattori, Akira;Kakeya, Hideaki [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 6,p. 1608 - 1611] Location in patent:supporting information

75178-90-4
132 suppliers
$8.00/250mg
104-15-4
764 suppliers
$133.00/500g
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg

24424-99-5
869 suppliers
$13.50/25G
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg

2508-29-4
213 suppliers
$11.00/5g
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg

2508-29-4
213 suppliers
$11.00/5g

24424-99-5
869 suppliers
$13.50/25G

75178-90-4
132 suppliers
$8.00/250mg

98-59-9
626 suppliers
$9.00/5g
![TERT-BUTYL N-[5-(TOSYLOXY)PENTYL]CARBAMATE](/CAS/GIF/118811-34-0.gif)
118811-34-0
39 suppliers
$87.50/250mg