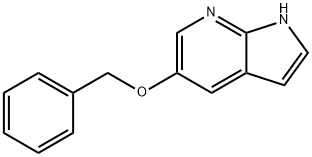
5-Benzyloxy-1H-pyrrolo[2,3-b]pyridine synthesis
- Product Name:5-Benzyloxy-1H-pyrrolo[2,3-b]pyridine
- CAS Number:1036007-71-2
- Molecular formula:C14H12N2O
- Molecular Weight:224.26
![1H-PYRROLO[2,3-B]PYRIDIN-5-OL](/CAS/GIF/98549-88-3.gif)
98549-88-3
302 suppliers
$8.00/250mg

100-39-0
430 suppliers
$10.00/10g
![5-Benzyloxy-1H-pyrrolo[2,3-b]pyridine](/CAS/20180702/GIF/1036007-71-2.gif)
1036007-71-2
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium phosphate in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
63 Reference embodiment 63: Preparation of intermediate 1-65
1H-pyrrolo[2,3-b]pyridin-5-ol (5.0 g, 37.3 mmol) was dissolved in N,N-dimethylformamide (60 mL). Benzyl bromide (9.6 g, 55.9 mmol) and potassium phosphate (15.8 g, 74.6 mmol) were added to the reaction system successively. The reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was slowly poured into water (300 mL) and extracted with ethyl acetate (50 mL×4). The combined organic phases were washed with saturated saline, dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove the organic solvent to obtain a residue. The residue was purified by silica gel chromatography to obtain intermediate 1-65. 1H NMR (400 MHz, CDCl3) δ 9.26 (s, 1H), 8.16 (d, J = 2.6Hz, 1H), 7.51 (d, J = 2.6 Hz, 1H), 7.50 - 7.43 (m, 2H), 7.43 - 7.37 (m, 2H), 7.37 - 7.28 (m, 2H), 6.48 - 6.39 (m, 1H), 5.14 (s, 2H).
References:
EP3892616,2021,A1 Location in patent:Paragraph 0185-0186
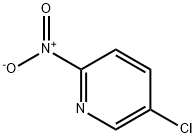
52092-47-4
357 suppliers
$6.00/1g
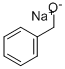
20194-18-7
32 suppliers
$131.00/25ml
![5-Benzyloxy-1H-pyrrolo[2,3-b]pyridine](/CAS/20180702/GIF/1036007-71-2.gif)
1036007-71-2
4 suppliers
inquiry