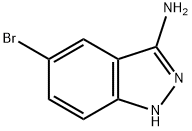
5-BROMO-1H-INDAZOL-3-AMINE synthesis
- Product Name:5-BROMO-1H-INDAZOL-3-AMINE
- CAS Number:61272-71-7
- Molecular formula:C7H6BrN3
- Molecular Weight:212.05
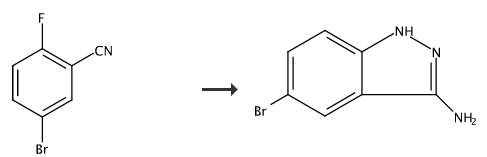
Hydrazine hydrate (18 mL) was added to Example 62C (1.93 g, 6.94 mmol.) in ethanol (10 mL). The mixture was heated to 95°C. overnight. The mixture was diluted with ethyl acetate and washed with water. Some of the product precipitated in the separatory funnel and was filtered to afford the title compound. The ethyl acetate layer was concentrated under reduced pressure and the resulting solid was triturated with methanol. 5-bromo-1H-indazol-3-amine. The title compound was prepared according to the procedure outlined in Example 62D substituting 5-bromo-2-fluorobenzonitrile for Example 62C. 1H NMR (400 MHz, DMSO-d6) δ ppm 11.55 (s, 1H), 7.92 (d, J=1.87 Hz, 1H), 7.30 (dd, J=8.79, 1.89 Hz, 1H), 7.19 (d, J=8.78 Hz, 1H), 5.41 (s, 2H).
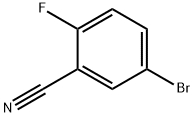
179897-89-3
317 suppliers
$10.00/5g

61272-71-7
146 suppliers
$9.00/250mg
Yield:61272-71-7 99.5%
Reaction Conditions:
with hydrazine at 100; for 0.0833333 h;
Steps:
45
To a 250 mL round-bottom flask was added 5-bromo-2-fluorobenzonitrile (15.54 g, 77.7 mmol) and hydrazine (124 g, 3885 mmol). The reaction mixture was heated to 100° C. for 5 minutes. The hydrazine was then removed under reduced pressure to give 5-bromo-1H-indazol-3-amine (16.4 g, 99.5% yield). MS m/z: 213 (M+1).
References:
US2007/173506,2007,A1 Location in patent:Page/Page column 47
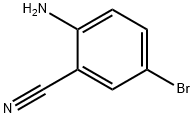
39263-32-6
243 suppliers
$15.00/1g

61272-71-7
146 suppliers
$9.00/250mg