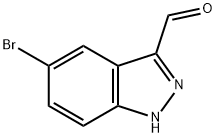
5-BROMO-1H-INDAZOLE-3-CARBALDEHYDE synthesis
- Product Name:5-BROMO-1H-INDAZOLE-3-CARBALDEHYDE
- CAS Number:201227-38-5
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04

705264-93-3

201227-38-5
Step 3: (5-bromo-1H-indazol-3-yl)methanol (0.267 g, 1.18 mmol) was dissolved in ethyl acetate (12 mL) and manganese dioxide (1.03 g, 11.8 mmol) was added. The reaction mixture was stirred at room temperature overnight. After completion of the reaction, solid impurities were removed by diatomaceous earth filtration and the filtrate was concentrated under reduced pressure to afford 5-bromo-1H-indazole-3-carbaldehyde (0.179 g, 67% yield). The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 7.63 (dd, J = 1.5, 8.8 Hz, 1H), 7.71 (d, J = 8.8 Hz, 1H), 8.27 (d, J = 1.5 Hz, 1H), 10.18 (s, 1H), 14.37 (br s, 1H).
10075-50-0
689 suppliers
$5.00/10g

201227-38-5
151 suppliers
$45.00/50mg
Yield:201227-38-5 76%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water;acetone at -10 - 20; for 3 h;
Steps:
1
A solution of NaNO2 (110.4 g, 1.6 mol, 8 eq) in water (200 mL) was added dropwise to a solution of 5-bromoindole (XI) (39.2 g, 0.2 mol, 1 eq) in acetone (1000 mL) stirred at -10→0° C., while adding NaNO2 the solution temperature was maintained below 20° C. An aqueous 2N HCl solution (480 mL) was added slowly to the solution with vigorously stirring while keeping the internal temperature between 0 and 20° C. The solution was further stirred at 20° C. for 3 h after the addition. The solution was concentrated under reduced pressure to remove acetone while keeping the temperature below 35° C. The solid was collected by filtration and transferred to a flask. Cold (-10° C.) DCM (200 mL) was added and stirred for 30 min at -5° C., the solids were filtered and dried under vacuum at 40° C. to get 5-bromo-1H-indazole-3-carbaldehyde (XII) (34.0 g, 151 mmol, 76% yield) as a brown solid. ESIMS found for C8H5BrN2O m/z 225 (M+H).
References:
Samumed, LLC;KC, Sunil Kumar;Wallace, David Mark;Cao, Jianguo;Chiruta, Chandramouli;Hood, John US2016/75701, 2016, A1 Location in patent:Paragraph 0798; 0799

10075-50-0
689 suppliers
$5.00/10g

67-64-1
0 suppliers
$17.30/10ml

201227-38-5
151 suppliers
$45.00/50mg

705264-93-3
20 suppliers
inquiry

201227-38-5
151 suppliers
$45.00/50mg

10075-50-0
689 suppliers
$5.00/10g

75-09-2
1263 suppliers
$10.00/25 mL

201227-38-5
151 suppliers
$45.00/50mg
78155-74-5
117 suppliers
$9.00/100mg

201227-38-5
151 suppliers
$45.00/50mg