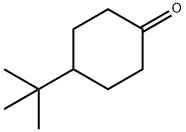
4-tert-Butylcyclohexanone synthesis
- Product Name:4-tert-Butylcyclohexanone
- CAS Number:98-53-3
- Molecular formula:C10H18O
- Molecular Weight:154.25
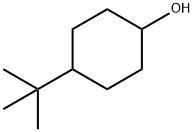
98-52-2
374 suppliers
$9.00/25g

98-53-3
272 suppliers
$5.00/1g
Yield:98-53-3 100%
Reaction Conditions:
with pyridine;trichloroisocyanuric acid in ethyl acetate at 20; for 0.333333 h;Reagent/catalyst;
Steps:
Oxidation of 4-t-butylcyclohexanaol
General procedure: O.1 mole of 4-t-butylcyclohexanol (ACROS) was weighed and placed in a 50 mL Erlenmeyer flask with stir bar and 10 mL ethyl acetate (Fisher). 1.0 g trichloroisocyanuric acid (Leslie’s Swimming Pool Supplies) was weighed into a 30 mL beaker and dissolved in 10 mL ethyl acetate. 1.0 mL (0.12 mol) pyridine (Aldrich) was delivered to the alcohol mixture by pipet. The TCICA solution was transferred to a dropping funnel and added dropwise to the stirring alcohol solution. Following addition, stirring was continued for 20 minutes and the mixture was filtered. The filtrate was washed with 10 mL 1 M HCl, 10 mL 5% NaHCO3, and 5 mL saturated NaCl. The organic layer was dried over MgSO4, a GC sample removed, filtered and evaporated to dryness. Yield 1.50 g (100%), nmr attached.
References:
Dip, Irene;Gethers, Christina;Rice, Tonya;Straub, Thomas S. [Tetrahedron Letters,2017,vol. 58,# 28,p. 2720 - 2722] Location in patent:supporting information
98-54-4
424 suppliers
$15.00/25g

98-53-3
272 suppliers
$5.00/1g
![9-(1,1-dimethylethyl)-1,5-dioxaspiro[5.5]undecane](/CAS/GIF/65156-97-0.gif)
65156-97-0
2 suppliers
inquiry

98-53-3
272 suppliers
$5.00/1g
![9-tert-butyl-3,3-dimethyl-1,5-dioxaspiro[5.5]undecane](/CAS/20180723/GIF/13483-96-0.gif)
13483-96-0
1 suppliers
inquiry

98-53-3
272 suppliers
$5.00/1g
937-07-5
5 suppliers
inquiry

98-53-3
272 suppliers
$5.00/1g