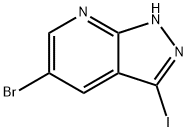
5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine synthesis
- Product Name:5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine
- CAS Number:875781-18-3
- Molecular formula:C6H3BrIN3
- Molecular Weight:323.92
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
General procedure for the synthesis of 5-bromo-3-iodo-1H-pyrazolo[3,4-B]pyrimidines (IV-A-I) from 5-bromo-1H-pyrazolo[3,4-B]pyridines: to a stirred solution of 5-bromo-1H-pyrazolo[3,4-B]pyridines (III-A-1) (1g, 5.05mmol) in DMF (14mL) was added in one go crushed KOH pellets (1.06 g, 19.03 mmol). after 11 min, I2 (1.15 g, 4.54 mmol) was added and the mixture was stirred vigorously for 3.5 h. The mixture was then partially purified in vacuo. The mixture was then partially concentrated in vacuo, diluted with EtOAc (40 mL) and saturated NaHCO3 solution (20 mL) and partitioned. The aqueous layer was extracted with EtOAc (2 x 20 mL). The combined organic layers were dried (MgSO4), filtered and concentrated to give a 1:1 mixture of III-A-1 and IV-A-1 (1.377 g). The mixture was redissolved in 1,4-dioxane (14 mL) and treated with solid NaOH (0.7 g). The mixture was stirred at room temperature for 5 min and I2 (0.7 g) was added. The mixture was stirred at 40 °C for 23 h. The mixture was diluted with EtOAc (50 mL) and washed with saturated aqueous Na2S2O3 (30 mL). The aqueous layer was extracted with EtOAc (2 x 30 mL), and the combined organic extracts were dried (MgSO4), filtered and concentrated to afford 5-bromo-3-iodo-1H-pyrazolo[3,4-B]pyrimidine (IV-A-I) (1.585 g, 97%).1H NMR (400 MHz; CDCl3) δ 7.94 (d, J=2.1 Hz, 1H) 8.55 (d, J=2.1Hz, 1H), 10.85 (brs, 1H, NH).
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
124 suppliers
$16.00/100mg
Yield: 97%
Reaction Conditions:
Stage #1:5-bromo-7-azaindazole with iodine;potassium hydroxide in N,N-dimethyl-formamide for 3.5 h;
Stage #2: with iodine;sodium hydroxide in 1,4-dioxane at 40; for 23 h;
Steps:
5
5-bromo-3 -iodo- 1 H-pyrazolo [3 ,4-bipyridine ( IV-A-I); To a stirred solution of the bromo-diazaindole III- A-I (1 g, 5.05 mmol) in DMF (14 mL) was added crushed KOH pellets (1.06 g, 19.03 mmol) in one portion. After 11 min, I2 (1.15 g, 4.54 mmol) was added and the mixture stirred vigorously for 3.5 h. The mixture was then partially concentrated in vacuo, diluted with EtOAc (40 mL): saturated NaHCO3 solution (20 mL) and partitioned. The aqueous layer was extracted with EtOAc (2 x 20 mL). The combined organic layers were dried (MgSO4), filtered and concentrated to afford a 1 :1 mixture of III-A-1 and IV-A-I (1.377 g). This mixture was re-dissolved in 1,4-dioxane (14 mL) and treated with solid NaOH (0.7 g). The mixture was stirred for 5 min at RT and I2 (0.7 g) was added. The mixture was stirred at 40 0C for 23 h, diluted with EtOAc (50 mL) and washed with saturated aqueous Na2S2O3 solution (30 mL). The aqueous layer was extracted with EtOAc (2 x 30 mL) and the combined organic extracts were dried (MgSO4), filtered and concentrated to afford azaindazole IV-A-I (1.585 g, 97%). 1H NMR (400 MHz; CDCl3) δ 7.94 (d, J2.1, IH), 8.55 (d, J2.1, IH), 10.85 (brs, NH).
References:
EISAI R & D MANAGEMENT CO. LTD;GRACZYK, Piotr;BHATIA, Gurpreet, Singh WO2010/15803, 2010, A1 Location in patent:Page/Page column 43
117007-52-0
147 suppliers
$13.00/1g
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
124 suppliers
$16.00/100mg
![1H-PYRAZOLO[3,4-B]PYRIDINE](/CAS/GIF/271-73-8.gif)
271-73-8
223 suppliers
$6.00/1g
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
124 suppliers
$16.00/100mg
516-12-1
573 suppliers
$5.00/5g
![5-BROMO-1H-PYRAZO[3,4-B]PYRIDINE](/CAS/GIF/875781-17-2.gif)
875781-17-2
260 suppliers
$7.00/250mg
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
124 suppliers
$16.00/100mg
875781-15-0
182 suppliers
$6.00/1g
![5-bromo-3-iodo-1H-pyrazolo[3,4-b]pyridine](/CAS/GIF/875781-18-3.gif)
875781-18-3
124 suppliers
$16.00/100mg