
5-broMo-2-(2-Methoxyethoxy)pyridine synthesis
- Product Name:5-broMo-2-(2-Methoxyethoxy)pyridine
- CAS Number:212961-29-0
- Molecular formula:C8H10BrNO2
- Molecular Weight:232.07
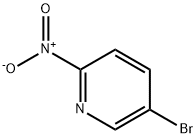
39856-50-3
544 suppliers
$6.00/5g

109-86-4
573 suppliers
$11.00/25g

212961-29-0
32 suppliers
$75.00/250mg
Yield:212961-29-0 86%
Reaction Conditions:
with sodium for 2.5 h;Reflux;
Steps:
5-Bromo-2-(2-methoxyethoxy)pyridine (17-2).
To a stirred solution of sodium (591 mg, 24.6 mmol) in 2-methoxyethanol (20 mL) was added a solution of 5-bromo-2-nitropyridine (17-1, 5.00 g, 24.6 mmol) in 2-methoxyethanol (10 mL) at room temperature. The reaction mixture was stirred at reflux for 2.5 h. The solution was concentrated and the residue was diluted with CH2CI2 and H2O. The organic layer was dried and evaporated under reduced pressure to give 5-bromo-2-(2-methoxyethoxy)pyridine (17-2, 4.9 g) in 86% yield: 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 2. 6 Hz, 1 H), 7.64 (dd, J = 8.7, 2.6 Hz, 1 H), 6.72 (d, J = 8.7 Hz, 1 H), 4.43 (t, J = 6.6 Hz, 2 H), 3.73 (t, 2 H), 3.41 (s, 3 H). ESI-MS: m/z 232.0 (M + H)+.
References:
WO2013/82324,2013,A1 Location in patent:Paragraph 00156
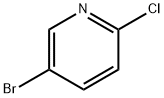
53939-30-3
482 suppliers
$5.00/1g

109-86-4
573 suppliers
$11.00/25g

212961-29-0
32 suppliers
$75.00/250mg
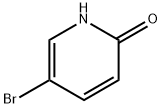
13466-38-1
398 suppliers
$6.00/5g

109-86-4
573 suppliers
$11.00/25g

212961-29-0
32 suppliers
$75.00/250mg
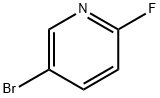
766-11-0
452 suppliers
$6.00/5g

109-86-4
573 suppliers
$11.00/25g

212961-29-0
32 suppliers
$75.00/250mg
624-28-2
720 suppliers
$5.00/5g

109-86-4
573 suppliers
$11.00/25g

212961-29-0
32 suppliers
$75.00/250mg