
5-BROMO-2,3-DIFLUORO-BENZALDEHYDE synthesis
- Product Name:5-BROMO-2,3-DIFLUORO-BENZALDEHYDE
- CAS Number:633327-22-7
- Molecular formula:C7H3BrF2O
- Molecular Weight:221
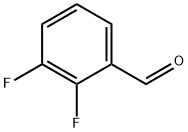
2646-91-5

633327-22-7
Under stirring conditions, 2,3-difluorobenzaldehyde (XVIII) (75.0 g, 528 mmol, 1.0 eq.) was dissolved in concentrated sulfuric acid (565 mL) and heated to 60°C. The mixture was then dissolved in a mixture of 2,3-difluorobenzaldehyde (XVIII) and 2,3-difluorobenzaldehyde (XVIII). Subsequently, N-bromosuccinimide (NBS) (113 g, 633 mmol, 1.2 eq.) was added in batches. The reaction mixture was stirred continuously at 60 °C for 12 h. The completion of the reaction was confirmed by LC/MS monitoring. The reaction mixture was slowly poured into a mixture of ice water (500 mL) and petroleum ether (500 mL) and stirred for 10 min before separating the organic layer. The organic layer was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: 100% petroleum ether) to afford 5-bromo-2,3-difluorobenzaldehyde (XIX) (120 g, 543.0 mmol, quantitative yield). The product was analyzed by electrospray ionization mass spectrometry (ESIMS) and the molecular formula was confirmed as C7H3BrF2O, m/z 221.1 ([M+H]+).

2646-91-5
299 suppliers
$5.00/250mg

633327-22-7
82 suppliers
$10.00/100mg
Yield:633327-22-7 100%
Reaction Conditions:
with N-Bromosuccinimide;sulfuric acid at 60; for 12 h;
Steps:
1 Step 1
j0617j To a stirred solution of 2,3-difluorobenzaldehyde (XVIII) (75.0 g, 528 mmol, 1.0 eq) in H2S04 (565 mL) was added NBS (113 g, 633 mmol, 1.2 eq) in portions at 60 °C. The resulting mixture was stirred at 60 °C for 12 hr. LC/MS showed the reaction was completed. The reaction mixture was poured into ice water and petroleum ether (500 mL) and stirred for 10 mm, the organic layer was separated and concentrated under vacuum to give cmde product. The residue was purified column chromatography silica gel (100% petroleum ether) to give 5-bromo-2,3- difluorobenzaldehyde (XIX) (120 g, 543.0 mmol, quantitative yield). ESIMS found C7H3BrF2O mlz 221.1 (M+1).
References:
WO2017/24021,2017,A1 Location in patent:Paragraph 0616; 0617
633327-21-6
4 suppliers
inquiry

633327-22-7
82 suppliers
$10.00/100mg