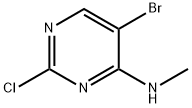
(5-BroMo-2-chloro-pyriMidin-4-yl)-Methyl-aMine synthesis
- Product Name:(5-BroMo-2-chloro-pyriMidin-4-yl)-Methyl-aMine
- CAS Number:205672-24-8
- Molecular formula:C5H5BrClN3
- Molecular Weight:222.47
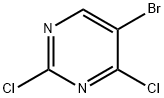
36082-50-5
489 suppliers
$5.00/1g

74-89-5
0 suppliers
$13.44/25ML

205672-24-8
28 suppliers
$220.00/100mg
Yield:205672-24-8 100%
Reaction Conditions:
in methanol at 0 - 20; for 3.66667 h;
Steps:
1 5.1.1 5-Bromo-2-chloro-N-methyl-pyrimidin-4-amine (3a)
Methylamine (9.8 M MeOH solution) (8.9 ml, 87.7 mmol) was added dropwise at 0 °C over 20 min to a solution of 5-bromo-2,4-dichloropyrimidine (10 g, 43.9 mmol) in MeOH (150 ml). After stirring for 20 min at 0 °C, the mixture was warmed to room temperature, stirred for 3 h, and evaporated down. The residue was suspended in EtOAc, washed with satd NaHCO3 and brine, dried with MgSO4, and evaporated down. The residue was chromatographed on silica gel column to give 10 g of 3a in quantitative yield. Rf = 0.15 (n-hexane/EtOAc = 5:1). 1H NMR (600 MHz, CDCl3): δ 8.12 (s, 1H), 5.58 (br s, 1H), 3.10 (d, J = 4.8 Hz, 3H).
References:
Teno, Naoki;Gohda, Keigo;Wanaka, Keiko;Tsuda, Yuko;Sueda, Takuya;Yamashita, Yukiko;Otsubo, Tadamune [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 7,p. 2339 - 2352]