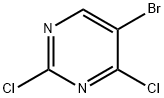
5-Bromo-2,4-dichloropyrimidine synthesis
- Product Name:5-Bromo-2,4-dichloropyrimidine
- CAS Number:36082-50-5
- Molecular formula:C4HBrCl2N2
- Molecular Weight:227.87
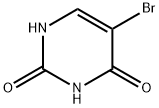
51-20-7

36082-50-5
General procedure for the synthesis of 5-bromo-2,4-dichloropyrimidine from 5-bromouracil: 5-bromouracil (6.0 g, 31.4 mmol) was placed in a reaction flask with phosphorus pentachloride (PCl5, 16.4 g, 87.9 mmol) and 1,1,2-trichloroethane (50 mL) was added as solvent. The reaction mixture was heated to reflux and the transformation of the mixture from suspension to a light yellow transparent solution was observed. The reaction progress was monitored by thin layer chromatography (TLC) and after confirming the complete conversion of the feedstock, the reaction mixture was cooled to room temperature. Subsequently, the reaction mixture was rapidly poured into stirred ice water and stirring was continued for 1 hour. The aqueous phase was extracted with dichloromethane (DCM, 3 x 50 mL), the organic layers were combined and dried with anhydrous magnesium sulfate (MgSO4). The solvent was removed by evaporation under reduced pressure to give a light yellow transparent liquid crude product. Finally, the crude product was purified by silica gel column chromatography to obtain the target compound 5-bromo-2,4-dichloropyrimidine as a colorless transparent liquid (99.5% yield, 97% purity).

51-20-7
455 suppliers
$10.00/1g

36082-50-5
490 suppliers
$5.00/1g
Yield:36082-50-5 99.5%
Reaction Conditions:
with phosphorus pentachloride in 1,1,2-trichloroethane;Reflux;Reagent/catalyst;Solvent;
Steps:
4 Example 4
5-bromouracil (6.0 g, 31.4 mmol) was mixed with PCl5 (16.4 g, 87.9 mmol) in a reaction flask.Add 1,1,2-trichloroethane (50 mL),The reaction mixture was heated to reflux.During the reaction, the mixture changed from a suspended state to a pale yellow clear solution.At this point TLC showed that the starting material was completely reacted.Drop to room temperature.Slowly pour the reaction mixture into stirred ice water.Stir for 1h,Add DCM (3 x 50 mL) for extraction.The organic layer was dried over anhydrous MgSO4.Evaporate the solvent,A pale yellow transparent liquid was obtained.Further purified on a silica gel column.Obtaining a colorless transparent liquid,That is, Compound 2 (yield 99.5%, purity 97%).
References:
CN108117523,2018,A Location in patent:Paragraph 0039; 0041; 0043; 0044; 0045

51-20-7
455 suppliers
$10.00/1g

36082-50-5
490 suppliers
$5.00/1g
113138-12-8
2 suppliers
inquiry

36082-50-5
490 suppliers
$5.00/1g