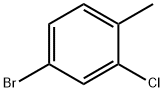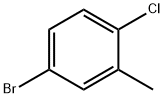
5-BROMO-2-CHLOROTOLUENE synthesis
- Product Name:5-BROMO-2-CHLOROTOLUENE
- CAS Number:54932-72-8
- Molecular formula:C7H6BrCl
- Molecular Weight:205.48
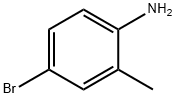
583-75-5

54932-72-8
General procedure for the synthesis of 5-bromo-2-chlorotoluene from 2-methyl-4-bromoaniline: 11.7 g (63.2 mmol) of 2-methyl-4-bromoaniline was dissolved in 45 mL (6 mol/L) of hydrochloric acid solution and stirred thoroughly to form the hydrochloride salt. The resulting solution was cooled to 0°C in an ice-salt bath. Under the condition that the temperature was kept below 0°C, about 20% sodium nitrite solution (4.8 g, 69.5 mmol, 20 mL) was added slowly and dropwise. Subsequently, 12 mL of 1,4-dioxane was added and stirred well. The diazonium salt solution prepared above was slowly added to a hydrochloric acid solution (20 mL) of 3 mol/L cuprous chloride (6.7 g, 66.4 mmol) in an ice-water bath with vigorous stirring. After completion of the dropwise addition, the reaction mixture was stirred at room temperature for 0.5 h and then at 80 °C for 3 h. The reaction mixture was then stirred for 0.5 h at room temperature. After completion of the reaction, the mixture was cooled and extracted with dichloromethane (75 mL x 2). The organic phases were combined, washed sequentially with NaHCO3 solution (150 mL), water (150 mL), and saturated brine (100 mL x 2), and dried with anhydrous sodium sulfate overnight. Finally, the dried organic phase was concentrated under reduced pressure to afford 11.2 g (87% yield) of reddish brown liquid product.

583-75-5
312 suppliers
$5.00/1g

54932-72-8
217 suppliers
$6.00/1g
Yield:54932-72-8 87%
Reaction Conditions:
with hydrogenchloride;copper(l) chloride;sodium nitrite in 1,4-dioxane;water at -5 - 80; for 3.5 h;
Steps:
1.2 Preparation of 5-bromo-2-chlorotoluene
11.7 g (63.2 mmol) of 4-bromo-2-methylaniline was added to 45 ml (6 mol / L) hydrochloric acid solution and stirred well to make the salt.The resulting solution was placed in an ice-salt bath, cooled to below -5 ° C, and about 20% sodium nitrite solution (4.8 g, 69.5 mmol, 20 ml) was slowly added dropwise to maintain the temperature below 0 ° C.Drop, add 12ml of 1,4-dioxane, stir well.The above diazonium salt solution was slowly added to a 3 mol / L hydrochloric acid solution (20 ml) of cuprous chloride (6.7 g, 66.4 mmol) in an ice-water bath and stirred vigorously. After completion of the dropwise addition, the mixture was stirred at room temperature for 0.5 h, To 80 ° C for 3 h.Cooled, extracted with dichloromethane (75 ml * 2) and the extract was saturated with NaHCO3(150 ml), water (150 ml) and saturated brine (100 ml * 2), dried over anhydrous sodium sulfate overnight and concentrated under reduced pressure to give 11.2 g (yield 87%) of reddish brown liquid.
References:
China Pharmaceutical University;XUE, XIAO WEN;LIU, LIN YI;LONG, JIN JIE;SONG, YA JING;LI, JIA BIN CN104478670, 2016, B Location in patent:Paragraph 0027-0028
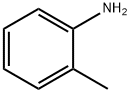
95-53-4
461 suppliers
$10.00/1g

54932-72-8
217 suppliers
$6.00/1g
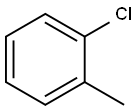
95-49-8
350 suppliers
$10.00/5g

54932-72-8
217 suppliers
$6.00/1g
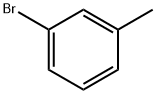
591-17-3
256 suppliers
$10.00/10g

54932-72-8
217 suppliers
$6.00/1g