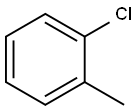
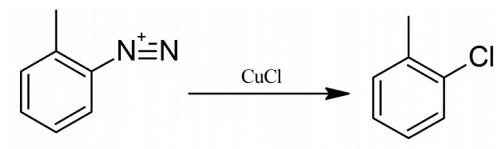
2-Chlorotoluene synthesis
- Product Name:2-Chlorotoluene
- CAS Number:95-49-8
- Molecular formula:C7H7Cl
- Molecular Weight:126.58
Experiments in Organic Chemistry, L. F. Fieser, 216, 1941
Highly Selective Oxidative Monochlorination To Synthesize Organic Intermediates: 2-Chlorotoluene, 2-Chloroaniline, 2-Chlorophenol, and 2-Chloro-4-methylphenol
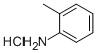
636-21-5
115 suppliers
$14.00/5g

95-49-8
350 suppliers
$10.00/5g
Yield:95-49-8 87%
Reaction Conditions:
Stage #1: o-toluidine hydrochloridewith isopentyl nitrite in acetone at 0 - 15;
Stage #2: with copper dichloride in ethanol;acetone at 5 - 20; for 2 h;Sandmeyer Reaction;Reagent/catalyst;Solvent;Temperature;
Steps:
1 Example 1
To a 100 ml three-necked flask was added 7.2 g of 2-methylaniline hydrochloride, 30 ml of acetone, and the ice-water bath was cooled to 0°C.8.8g of isoamyl nitrite was added dropwise, the temperature of the dropping process system was increased, the dropping rate was controlled, and the temperature was controlled at 0-15°C.Upon completion of the addition, 2-methylaniline hydrochloride dissolved and the system appeared pale yellow.5.0 g of copper chloride was dissolved in 30 ml of ethanol and added dropwise to the above system to control the dropping rate and the temperature was lower than 5°C.During the dropwise addition, yellow solids precipitated in the system, and the color deepened. The viscosity of the system became viscous with the addition of one third, and the reaction system was yellow-green with the dropwise addition of the copper chloride ethanol solution. After the addition was completed, the reaction was carried out in an ice bath for 20 minutes, gradually warmed to room temperature and reacted for 2 hours, and the system turned from yellow-green to dark green.Using 50ml of toluene, spin-degassing, precipitation of green solids, filtration, 5.0g; distillation,154-158°C distillate 5.5g was collected with a yield of 87%.
References:
CN107805180,2018,A Location in patent:Paragraph 0034-0038; 0042-0044
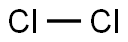
7782-50-5
0 suppliers
$405.00/454g
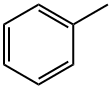
108-88-3
0 suppliers
$14.00/100ml
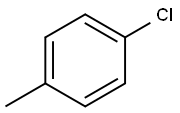
106-43-4
356 suppliers
$14.00/5g

95-49-8
350 suppliers
$10.00/5g