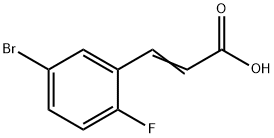
5-BROMO-2-FLUOROCINNAMIC ACID synthesis
- Product Name:5-BROMO-2-FLUOROCINNAMIC ACID
- CAS Number:202865-71-2
- Molecular formula:C9H6BrFO2
- Molecular Weight:245.05
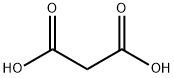
141-82-2
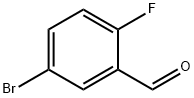
93777-26-5

202865-71-2
A Knoevenagel condensation reaction catalyzed by piperidine (0.5 mL, 4.9 mmol) in pyridine (11.4 mL) was carried out with 5-bromo-2-fluorobenzaldehyde (5.0 g, 24.6 mmol) and malonic acid (5.6 g, 53.7 mmol). The reaction mixture was stirred at 100 °C for 3 h. After completion of the reaction, the solvent was removed by concentration under reduced pressure. Distilled water was added to the concentrated mixture and the precipitate was collected by filtration. The resulting crude product was purified by recrystallization with ethanol to give the final 5-bromo-2-fluorocinnamic acid (1.3 g, white solid). The structure of the product was confirmed by 1H NMR (400 MHz, CD3OD): δ 7.89 (d, 1H), 7.74-7.70 (m, 2H), 7.15 (t, 1H), 6.62 (d, 1H).

141-82-2
789 suppliers
$5.00/25g

93777-26-5
371 suppliers
$5.00/5g

202865-71-2
101 suppliers
$6.00/1g
Yield: 1.3 g
Reaction Conditions:
with piperidine;pyridine at 100; for 3 h;
Steps:
8.1 Step 1: 3-(5-bromo-2-fluoro-phenyl)-acrylic acid
5-Bromo-2-fluoro-benzaldehyde (5.0 g, 24.6 mmol), malonic acid (5.6 g, 53.7 mmol), and piperidine (0.5 mL, 4.9 mmol) were added to pyridine (11.4 mL). The reaction mixture was stirred at 100° C. for 3 hours and then concentrated under reduced pressure. Distilled water was added to the reaction mixture, which was then filtered. The resulting solid was recrystallized from ethanol to give 1.3 g of the titled compound as a white solid. (0164) 1H NMR (400 MHz, CD3OD) 7.89 (d, 1H), 7.74-7.70 (m, 2H), 7.15 (t, 1H), 6.62 (d, 1H)
References:
YUHAN CORPORATION;Park, Chan-Sun;Kim, Young-Hwan;Lee, Gyu-Jin;Hur, Youn;Jung, Eun-Hye;Tak, Hee-Jae;Shin, Seung-Yub;Lee, Ho-Jin;Lee, Chun-Ho;Lee, Koo-Yeon US2015/291563, 2015, A1 Location in patent:Paragraph 0163; 0164