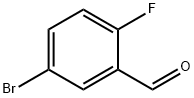
5-Bromo-2-fluorobenzaldehyde synthesis
- Product Name:5-Bromo-2-fluorobenzaldehyde
- CAS Number:93777-26-5
- Molecular formula:C7H4BrFO
- Molecular Weight:203.01
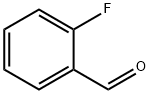
446-52-6

93777-26-5
Example 3: In a reactor equipped with an electric stirrer, a thermometer, a dropping funnel and a condenser tube, 500 mL of aqueous sulfuric acid solution was added. Subsequently, a mixture of 167 g (1 mol) of potassium bromate and 124.1 g (1 mol) of 2-fluorobenzaldehyde was slowly added dropwise to the reaction system, the reaction temperature was maintained at 90°C, and the dropwise process continued for 2 to 3 hours. Upon completion of the reaction, 1000 mL of water was added to the system and extracted using methyl tert-butyl ether. The organic phase was subsequently washed with aqueous sodium sulfite and dried over anhydrous sodium sulfate. The dried organic phase was filtered and concentrated to remove the methyl tert-butyl ether to give a brownish red oil. Finally, the 63-65°C/3 mmHg fraction was collected by vacuum distillation to afford 178 g of the target product 5-bromo-2-fluorobenzaldehyde in 88% yield and 97% purity.

446-52-6
438 suppliers
$6.00/25g

93777-26-5
371 suppliers
$5.00/5g
Yield:93777-26-5 88%
Reaction Conditions:
with potassium bromate;sulfuric acid in water at 90;Reagent/catalyst;Temperature;
Steps:
3
Example three: equipped with electric stirring, thermometer, Dropping funnel and condenser tube reactor was added 65% aqueous sulfuric acid 500ml, Potassium bromate 167 g (1 mol), 124.1 g (1 mol) of o-fluorobenzaldehyde was added dropwise thereto, 90 ° C for 2-3 hours, to the system by adding water 1000ml, Extraction with methyl tert-butyl ether, The organic phase was washed with aqueous sodium sulfite, dried over anhydrous sodium sulfate and concentrated by filtration to remove methyl tert-butyl ether to give a brown-red oil. Vacuum distillation collected 63-65 ° C / 3mmHg fraction 178g, Yield 88%, content 97%.
References:
Shanghai Zhiyan Pharmaceutical Technology Co., Ltd.;Wang Dongwei;Ma Jian;Ye Mao;Zhang Jiyong CN105884591, 2016, A Location in patent:Paragraph 0008; 0009; 0010; 0011
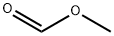
107-31-3
356 suppliers
$16.00/25mL
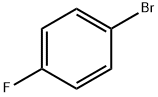
460-00-4
636 suppliers
$6.00/10g

93777-26-5
371 suppliers
$5.00/5g
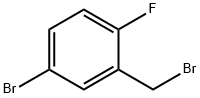
99725-12-9
131 suppliers
$11.00/1g

93777-26-5
371 suppliers
$5.00/5g
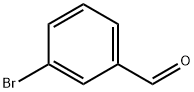
3132-99-8
507 suppliers
$5.00/10g

93777-26-5
371 suppliers
$5.00/5g