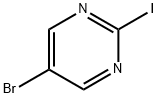
5-Bromo-2-iodopyrimidine synthesis
- Product Name:5-Bromo-2-iodopyrimidine
- CAS Number:183438-24-6
- Molecular formula:C4H2BrIN2
- Molecular Weight:284.88
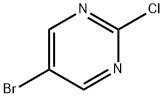
32779-36-5
631 suppliers
$5.00/5g

183438-24-6
389 suppliers
$6.00/1g
Yield:183438-24-6 84%
Reaction Conditions:
Stage #1:5-Bromo-2-chloropyrimidine with hydrogen iodide;sodium iodide in chloroform;water at 0 - 20; for 20 h;
Stage #2: with sodium hydroxide in chloroform;water for 0.166667 h;
Steps:
A Preparation of 5-Bromo-2-iodo-pyrimidine
Preparation of 5-Bromo-2-iodo-pyrimidine To a suspension of 5-bromo-2-chloro-pyrimidine (5.80 g, 30 mmol) and sodium iodide (7.5 g, 50 mmol) in chloroform (20 ml) a Hydroiodic acid (57 wt. %) (2.85 g, 25.6 mmol) is added at 0° C. After removing the cooling the reaction mixture is stirred for 20 h at r.t. and then poured into a mixture of 200 ml ice water and 30 ml 10N NaOH. Chloroform (150 ml) is added and the mixture is stirred for 10 min. The organic phase is separated, the aqueous layer is extracted with chloroform (2*100 ml) and the combined organic phases dried over MgSO4 and concentrated in vacuo to yielding 5-bromo-2-iodo-pyrimidine as a pale yellow solid. Yield 6.29 g (84%) MS: M=284.8 (ESI+) 1H-NMR (300 MHz, CDCl3): 8.54 (s, 2H) 7.56(d, J=16.4 Hz, 1H), 7.59-7.66(m, 4H).
References:
Bossenmaier, Birgit;Friebe, Walter-Gunar;Jenni, Wolfgang;Rueth, Matthias;Voss, Edgar US2005/222228, 2005, A1 Location in patent:Page/Page column 15-16
7752-82-1
428 suppliers
$13.00/25g

183438-24-6
389 suppliers
$6.00/1g
38353-09-2
404 suppliers
$5.00/5g

183438-24-6
389 suppliers
$6.00/1g

38353-09-2
404 suppliers
$5.00/5g

183438-24-6
389 suppliers
$6.00/1g