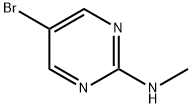
5-BROMO-2-(METHYLAMINO)PYRIMIDINE synthesis
- Product Name:5-BROMO-2-(METHYLAMINO)PYRIMIDINE
- CAS Number:31402-54-7
- Molecular formula:C5H6BrN3
- Molecular Weight:188.03
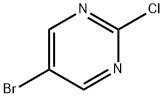
32779-36-5

74-89-5

31402-54-7
1. 5-Bromo-2-chloropyrimidine (3 g, 15.5 mmol), 40% aqueous methylamine (35 ml) and methanol (20 ml) were added to a reaction flask and mixed well with stirring. 2. The reaction mixture was heated to reflux and kept at that temperature for 3 days. 3. After completion of the reaction, the reaction was cooled to room temperature. 4. The solvent was removed by evaporation using a rotary evaporator under reduced pressure. 5. The residue was extracted by partitioning with dichloromethane and 1M sodium hydroxide. 6. The residue was extracted by partitioning with dichloromethane and 1 M sodium hydroxide solution. 6. The organic layer was separated and dried over anhydrous magnesium sulfate. 7. After filtration to remove the desiccant, the solvent was again evaporated under reduced pressure using a rotary evaporator to give the white solid product 5-bromo-2-methylaminopyrimidine (3.0 g, 100% yield). 8. The product was characterized by 1H-NMR (CDCl3): δ 2.98 (3H, d, J=5.12 Hz), 5.16 (1H, brs), 8.29 (2H, s).

32779-36-5
633 suppliers
$5.00/5g

74-89-5
0 suppliers
$13.44/25ML

31402-54-7
117 suppliers
$5.00/100mg
Yield: 100%
Reaction Conditions:
in methanol;water for 72 h;Reflux;
Steps:
18.1
40% Aqueous methylamine solution (35 ml) and methanol (20 ml) were added to 5-bromo-2-chloropyrimidine (3 g, 15.5 mmol) and the resulting mixture was heated to reflux for 3 days. The mixture was cooled, then the solvent was evaporated under reduced pressure, the residue was partitioned with methylene chloride and 1 M sodium hydroxide, the organic layer was dried over magnesium sulfate, and the solvent was evaporated under reduced pressure to give the title compound (3.0 g, 100%) as a white solid.1H-NMR (CDCl3) δ: 2.98 (3H, d, J=5.12 Hz), 5.16 (1H, brs), 8.29 (2H, s).
References:
DAIICHI SANKYO COMPANY, LIMITED US2010/130492, 2010, A1 Location in patent:Page/Page column 93
7752-82-1
428 suppliers
$13.00/25g

74-88-4
357 suppliers
$15.00/10g

31402-54-7
117 suppliers
$5.00/100mg
38696-21-8
122 suppliers
$16.00/1 g
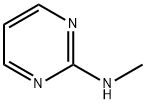
931-61-3
72 suppliers
$45.00/5mg

31402-54-7
117 suppliers
$5.00/100mg
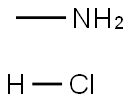
593-51-1
542 suppliers
$21.00/100g

32779-36-5
633 suppliers
$5.00/5g

31402-54-7
117 suppliers
$5.00/100mg