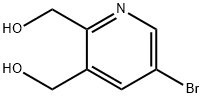
(5-BroMo-3-hydroxyMethyl-pyridin-2-yl)-Methanol synthesis
- Product Name:(5-BroMo-3-hydroxyMethyl-pyridin-2-yl)-Methanol
- CAS Number:1356330-71-6
- Molecular formula:C7H8BrNO2
- Molecular Weight:218.05
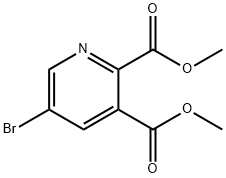
521980-82-5
59 suppliers
$72.00/250mg

1356330-71-6
34 suppliers
$45.00/10mg
Yield:1356330-71-6 65.84%
Reaction Conditions:
with sodium tetrahydridoborate;calcium(II) chloride in ethanol at -5 - 20; for 16 h;
Steps:
S135.b Step b)
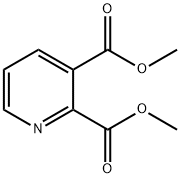
To a solution of dimethyl 5-bromopyridine-2, 3-dicarboxylate (42 g, 153.25 mmol) in EtOH (500 mL) was slowly added NaBH4(28.99 g, 766.23 mmol) at -5 . Then the CaCl2(15.31 g, 137.92 mmol) in EtOH (150 mL) was added dropwise slowly at -5 . The mixture was stirred for at 20 for 16 h. The mixture was quenched by slow addition of aqueous 2 N HCl solution (500 mL, pH2-3) . After stirring for 2 h, the mixture was concentrated to give the residue. Saturated aqueous sodium bicarbonate solution was added to the residue until pH=7. The aqueous mixture was extracted with EtOAc 900 mL (450 mL x 2) . The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give a crude product. The crude product was purified by column chromatography (SiO2, DCM: methanol: NH3·H2O= 50: 1: 0.1 to 10: 1: 0.1, plate 2) to give [5-bromo-2-(hydroxymethyl)-3-pyridyl]methanol (22 g, 100.90 mmol, 65.84%yield) as a light yellow solid.1H NMR (400 MHz, DMSO-d6) δ 4.53 (s, 2 H) , 4.64 (s, 2 H) , 7.99 (d, J=1.71 Hz, 1 H) , 8.50 (d, J=2.20 Hz, 1 H) . LC-MS: (ES) m/z 217.9 (M+H+) .
References:
WO2022/28586,2022,A1 Location in patent:Paragraph 0354; 0355
89-00-9
487 suppliers
$5.00/5g

1356330-71-6
34 suppliers
$45.00/10mg
98555-51-2
100 suppliers
$16.00/100mg

1356330-71-6
34 suppliers
$45.00/10mg
605-38-9
201 suppliers
$14.00/5g

1356330-71-6
34 suppliers
$45.00/10mg