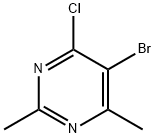
5-Bromo-4-chloro-2,6-dimethylpyrimidine synthesis
- Product Name:5-Bromo-4-chloro-2,6-dimethylpyrimidine
- CAS Number:69696-35-1
- Molecular formula:C6H6BrClN2
- Molecular Weight:221.48
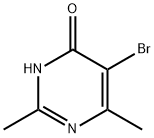
858269-28-0

69696-35-1
(b) Synthesis of 5-bromo-4-chloro-2,6-dimethylpyrimidine. 5-Bromo-2,6-dimethylpyrimidin-4-ol (3.95 g, 19.45 mmol) was mixed with phosphorochloridic acid (6 mL, 64.4 mmol) and heated to reflux for 3 h at 110 °C. The reaction was carried out at a pressure of 1.5 °F. Upon completion of the reaction, the reaction solution was concentrated under reduced pressure, followed by the slow and careful addition of ice water to precipitate a white precipitate. The solid was collected by filtration, washed thoroughly with cold water and dried to afford the target product 5-bromo-4-chloro-2,6-dimethylpyrimidine (2.73 g, 63% yield). The product was analyzed by LCMS (ES+), m/e 221 [M+H]+.

858269-28-0
26 suppliers
$45.00/50mg

69696-35-1
42 suppliers
$69.00/100mg
Yield:69696-35-1 63%
Reaction Conditions:
with trichlorophosphate at 110; for 3 h;
Steps:
56.b b) 5-bromo-4-chloro-2,6-dimethylpyrimidine
b) 5-bromo-4-chloro-2,6-dimethylpyrimidine. A mixture of 5-bromo-2,6- dimethylpyrimidin-4-ol (3.95 g, 19.45 mmol) and phosphorus oxychloride (6 mL, 64.4 mmol) was heated at 1 10 °C for 3h. The solution was concentrated, and water was then added slowly and cautiously to afford a precipitate. The solid was filtered and washed with water, and dried to give the title compound (2.73 g, 63%). LCMS (ES+) m/e 221 [M+H]+.
References:
WO2013/96151,2013,A1 Location in patent:Page/Page column 131
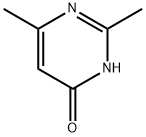
6622-92-0
206 suppliers
$9.00/1g

69696-35-1
42 suppliers
$69.00/100mg

858269-28-0
26 suppliers
$45.00/50mg

69696-35-1
42 suppliers
$69.00/100mg

6622-92-0
206 suppliers
$9.00/1g

69696-35-1
42 suppliers
$69.00/100mg