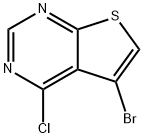
5-bromo-4-chlorothieno[2,3-d]pyrimidine synthesis
- Product Name:5-bromo-4-chlorothieno[2,3-d]pyrimidine
- CAS Number:814918-95-1
- Molecular formula:C6H2BrClN2S
- Molecular Weight:249.52
![6-BROMO-4-CHLOROTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/56844-12-3.gif)
56844-12-3
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
To a solution of diisopropylamine (1.028 mL, 7.21 mmol, 1.8 eq.) dissolved in 10 mL of THF at 0 °C was slowly added n-butyllithium (3.76 mL, 6.01 mmol, 1.5 eq.). After 1 hr of reaction, the resulting LDA solution was transferred to a 35 mL THF solution containing 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1.0 g, 4.01 mmol, 1.0 eq.) at -78 °C and under nitrogen protection. Stirring was continued at -78 °C for 1 h. A mixture consisting of 1.25 mL of water and 5 mL of THF was then slowly added. The reaction mixture was gradually warmed to 0 °C, poured into 60 mL of water and extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a yellow solid. The crude product was purified by silica gel column chromatography using a 20% ethyl acetate/hexane gradient elution to give 5-bromo-4-chlorothieno[2,3-d]pyrimidine (tan solid, 671 mg, 67.1% yield). NMR hydrogen spectrum (400 MHz, chloroform-d) δ 8.85 (s, 1H), 7.64 (s, 1H).
![6-BROMO-4-CHLOROTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/56844-12-3.gif)
56844-12-3
111 suppliers
$11.00/100mg
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
108 suppliers
$77.00/100mg
Yield: 67.1%
Reaction Conditions:
with n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Steps:
[0123] To diisopropylamine (1.028 ml, 7.21 mmol, 1.8 equiv) in 10 mL THF at 0 °C was added n-butyl lithium (3.76 ml, 6.01 mmol, 1.5 equiv). After 1 h, the LDA solution was transferred to a solution of 6-bromo-4-chlorothieno[2,3-d]pyrimidine (1.0 g, 4.01 mmol, 1.0 equiv) in 35 mL THF at -78 °C under nitrogen. The solution stirred for 1 h at -78 °C after which a mixture of 1.25 mL water and 5 mL THF was added slowly. The mixture was then warmed to 0 °C, poured into 60 mL water, and extracted with dichloromethane. The combined organic extracts were then dried over Na2SC> , filtered, and concentrated in vacuo to give a yellow solid which was chromatographed with 20% EtOAc/Hexanes gradient elution to give 5-bromo-4-chlorothieno[2,3-d]pyrimidine (671 mg, 67.1 % yield) as a tan solid. NMR (400 MHz, chloroform- ) δ ppm 8.85 (s, 1 H), 7.64 (s, 1 H).
References:
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES;MARUGAN, Juan, J.;ZHENG, Wei;SOUTHALL, Noel;HUANG, Wenwei;MCCOY, Joshua, G.;TITUS, Steven;PATNAIK, Samarjit WO2012/44993, 2012, A1 Location in patent:Page/Page column 44
![Thieno[2,3-d]pyrimidin-4(3H)one](/CAS/GIF/14080-50-3.gif)
14080-50-3
186 suppliers
$9.00/250mg
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
108 suppliers
$77.00/100mg
![Thieno[2,3-d]pyrimidin-4(3H)one](/CAS/GIF/14080-50-3.gif)
14080-50-3
186 suppliers
$9.00/250mg
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
108 suppliers
$77.00/100mg
4651-82-5
182 suppliers
$7.00/1g
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
108 suppliers
$77.00/100mg
![5,6-DibroMothieno[2,3-d]pyriMidin-4(3H)-one](/CAS/20150408/GIF/1239460-82-2.gif)
1239460-82-2
18 suppliers
inquiry
![5-bromo-4-chlorothieno[2,3-d]pyrimidine](/CAS/GIF/814918-95-1.gif)
814918-95-1
108 suppliers
$77.00/100mg