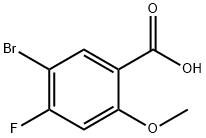
5-BroMo-4-fluoro-2-Methoxy-benzoic acid synthesis
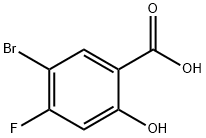
- Product Name:5-BroMo-4-fluoro-2-Methoxy-benzoic acid
- CAS Number:95383-26-9
- Molecular formula:C8H6BrFO3
- Molecular Weight:249.03
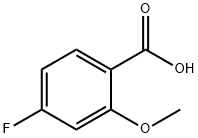
395-82-4

95383-26-9
(Step 1) 4-Fluoro-2-methoxybenzoic acid (1.70 g) was dissolved in acetic acid (20 mL) at room temperature and bromine (0.56 mL) was slowly added. The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction, water was added to the mixture and the precipitate was collected by filtration and washed with water and hexane. 5-Bromo-4-fluoro-2-methoxybenzoic acid (2.38 g, 96% yield) was obtained as a white powder.1H-NMR (300 MHz, CDCl3): δ 4.07 (3H, s), 6.86 (1H, d, J = 9.6 Hz), 8.39 (1H, d, J = 7.8 Hz).
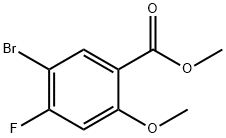
314298-22-1
47 suppliers
$45.00/100mg

95383-26-9
51 suppliers
$19.00/100mg
Yield:95383-26-9 97%
Reaction Conditions:
with lithium hydroxide monohydrate;sodium hydroxide in methanol at 20; for 15 h;
Steps:
Step C. Intermediate 114: 5-Bromo-4-fluoro-2-methoxybenzoic ac
A solution of NaOH (16.88 g, 421.95 mmol) in H2O (80 mL) was added slowly to a stirred solution of Intermediate 113 (37 g, 140.6 mmol) in MeOH (250 mL) at 20 °C. The resulting solution was stirred at 20 °C for 15 h. The reaction mixture was poured into ice/H2O (2 L), extracted with DCM (3 x 500 mL), the organic layer was dried over Na2SO4, filtered and evaporated to afford the title compound (34.0 g, 97%) as a white solid.1H NMR (300 MHz, CDCl3): δ 4.09 (s, 3 H), 6.88 (d, 1 H), 8.41 (d, 1 H). (ESI): m/z [M+H]+ 249.8.
References:
WO2022/122773,2022,A1 Location in patent:Page/Page column 191-192

395-82-4
169 suppliers
$19.00/1g

95383-26-9
51 suppliers
$19.00/100mg
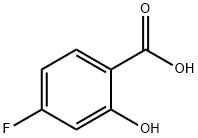
345-29-9
232 suppliers
$10.00/1g

95383-26-9
51 suppliers
$19.00/100mg
1644-71-9
42 suppliers
$103.00/250mg

95383-26-9
51 suppliers
$19.00/100mg