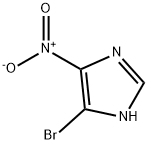
5-BROMO-4-NITRO-1H-IMIDAZOLE synthesis
- Product Name:5-BROMO-4-NITRO-1H-IMIDAZOLE
- CAS Number:6963-65-1
- Molecular formula:C3H2BrN3O2
- Molecular Weight:191.97
2302-25-2

6963-65-1
Example 312A Synthesis of 5-bromo-4-nitro-1H-imidazole: 4-bromo-1H-imidazole (2.0 g, 13.6 mmol) was mixed with concentrated nitric acid (0.947 mL, 14.96 mmol) in concentrated sulfuric acid (20 mL), and reacted for 1 hr at 110°C. After completion of the reaction, the mixture was cooled to 25 °C and slowly poured into 200 mL of ice water. The precipitated white solid was collected by filtration to give 5-bromo-4-nitroimidazole (2.3 g, 87% yield). Mass spectrum (ESI-) m/z 191 ([M-H]-). 1H NMR (300 MHz, DMSO-d6) δ 7.99 (s, 1H).

2302-25-2
256 suppliers
$6.00/1g

6963-65-1
80 suppliers
$53.00/1g
Yield: 87%
Reaction Conditions:
with nitric acid in (2S)-N-methyl-1-phenylpropan-2-amine hydrate;sulfuric acid
Steps:
312.A 5-Bromo-4-nitro-1H-imidazole
Example 312A 5-Bromo-4-nitro-1H-imidazole 4-Bromo-1H-imidazole (2.0 g, 13.6 mmol) was reacted with concentrated nitric acid (0.947 mL, 14.96 mmol) in concentrated sulfuric acid (20 mL) at 110° C. for 1 hour. The reaction was cooled to 25° C. and poured into 200 mL of ice water. The resulting white precipitate formed was collected by filtration to give the title compound (2.3 g, 87%). MS (ESI-) m/z 191 (M-H)-. 1H NMR (300 MHz, DMSO-d6) δ ppm 7.99 (s, 1H).
References:
Pratt, John K.;Betebenner, David A.;Donner, Pamela L.;Green, Brian E.;Kempf, Dale J.;McDaniel, Keith F.;Maring, Clarence J.;Stoll, Vincent S.;Zhang, Rong US2004/162285, 2004, A1

2302-25-2
256 suppliers
$6.00/1g

6963-65-1
80 suppliers
$53.00/1g