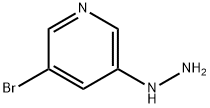
(5-Bromo-pyridin-3-yl)-hydrazine synthesis
- Product Name:(5-Bromo-pyridin-3-yl)-hydrazine
- CAS Number:801203-50-9
- Molecular formula:C5H6BrN3
- Molecular Weight:188.03
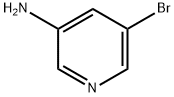
13535-01-8
381 suppliers
$3.00/1g

801203-50-9
15 suppliers
inquiry
Yield:801203-50-9 20%
Reaction Conditions:
Stage #1: 5-Bromo-pyridin-3-ylaminewith hydrogenchloride;NaNO2 in lithium hydroxide monohydrate at -5; for 1 h;
Stage #2: with hydrogenchloride;dichloro-λ2-stannane dihydrate in lithium hydroxide monohydrate at -5; for 2 h;
Steps:
(5-Bromo-3-pyridyl)hydrazine
To a cooled (-5 °C) solution of 5-bromopyridin-3-amine (1.00 g, 5.78 mmol) in water (15 mL) HCl (15 mL, 33 % wt) was added. A solution of NaNO2 (479 mg, 6.94 mmol) in water (3 mL) was added dropwise and the mixture was stirred 1 h at -5 °C. Tin(II) chloride dihydrate (3.26 g, 14.4 mmol) dissolved in concentrated HCl (5 mL) was added dropwise at -5 °C. The mixture was stirred 2 h at -5 °C. Then NaOH (100 mL, 10 % wt) solution was added dropwise at 0 °C to adjust pH to 9. Water (50 mL) and DCM (50 mL) were added. The aqueous phase was extracted with DCM (2 x 50 mL). The combined organic phases were washed with water (50 mL) and brine (2 x 50 mL), dried over anhydrous Na2SO4, evaporated and purified by column chromatography (PE/EtOAc = 1/0~3/1, silica-CS 40g 40 mL/min, silica gel, UV 254 nm) to afford the product (220 mg, 20 %). ESI-MS m/z calcd for [C5H6BrN3] [M+H]+: 188.0; found: 188.0.1H NMR (400 MHz, DMSO-d6) δ 8.01 (d, J = 2.4 Hz, 1H), 7.81 (d, J = 2.0 Hz, 1H), 7.32 - 7.31 (m, 2H), 4.28 (s, 2H).
References:
WO2022/144274,2022,A1 Location in patent:Page/Page column 151-152
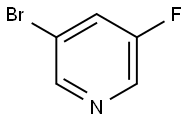
407-20-5
339 suppliers
$10.00/1g

801203-50-9
15 suppliers
inquiry
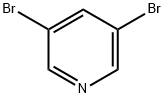
625-92-3
478 suppliers
$6.00/10g

801203-50-9
15 suppliers
inquiry