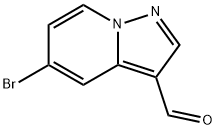
5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde synthesis
- Product Name:5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde
- CAS Number:1101120-53-9
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
![5-broMopyrazolo[1,5-a]pyridine](/CAS/20150408/GIF/1060812-84-1.gif)
1060812-84-1
130 suppliers
$60.00/100mg
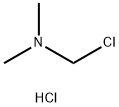
149409-22-3
3 suppliers
inquiry
![5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde](/CAS/20180703/GIF/1101120-53-9.gif)
1101120-53-9
9 suppliers
inquiry
Yield:1101120-53-9 100%
Reaction Conditions:
in dichloromethane at 44;
Steps:
3.2 5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde
To a solution of 5-bromopyrazolo[1,5-a]pyridine (175 mg, 0.89 mmol) in DCM (6 mL) was added (chloromethylene)dimethyliminium chloride (632 mg, 3.56 mmol). The reaction was stirred at 44° C. overnight, and concentrated in vacuo. The residue was dissolved in saturated NaHCO3 aqueous solution (25 mL) and the resulted mixture was then extracted with EtOAc (25 mL×3). The combined organic phases were dried over anhydrous Na2SO4 and concentrated in vacuo to give the title compound as a light yellow solid (225 mg, 100%). [0332] MS (ESI, pos. ion) m/z: 225.0 [M+H]+.
References:
US2014/234254,2014,A1 Location in patent:Paragraph 0331; 0332
![5-BROMO-PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/885276-93-7.gif)
885276-93-7
124 suppliers
$36.00/100mg
![5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde](/CAS/20180703/GIF/1101120-53-9.gif)
1101120-53-9
9 suppliers
inquiry
![ethyl 5-((tert-butoxycarbonyl)aMino)pyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/GIF/1101120-33-5.gif)
1101120-33-5
47 suppliers
$45.00/10mg
![5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde](/CAS/20180703/GIF/1101120-53-9.gif)
1101120-53-9
9 suppliers
inquiry
98400-69-2
172 suppliers
$6.00/1g
![5-bromopyrazolo[1,5-a]pyridine-3-carbaldehyde](/CAS/20180703/GIF/1101120-53-9.gif)
1101120-53-9
9 suppliers
inquiry