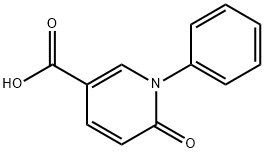
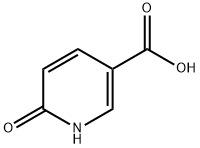
5-CARBOXY-N-PHENYL-2-1H-PYRIDONE synthesis
- Product Name:5-CARBOXY-N-PHENYL-2-1H-PYRIDONE
- CAS Number:77837-08-2
- Molecular formula:C12H9NO3
- Molecular Weight:215.2
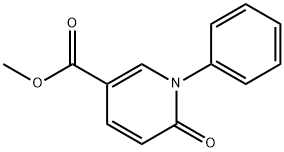
77837-09-3

77837-08-2
General procedure for the synthesis of 6-oxo-1-phenyl-1,6-dihydro-pyridine-3-carboxylic acid from methyl 6-oxo-1-phenyl-1,6-dihydro-pyridine-3-carboxylate: at 0 °C, lithium hydroxide monohydrate (0.366 g, 8.73 mmol) was added to a mixed solution of tetrahydrofuran (9 mL) and water (6 mL) containing methyl 6-oxo-1-phenyl-1,6-dihydropyridine-3-carboxylate (1.0 g, 4.37 mmol) in a mixed solution of tetrahydrofuran (9 mL) and water (6 mL) and the reaction was stirred for 1 hour. After completion of the reaction, the reaction mixture was diluted with water and washed with ethyl acetate. Subsequently, the pH of the aqueous layer was adjusted to 2 with 2N hydrochloric acid and the precipitate was collected by filtration to afford 6-oxo-1-phenyl-1,6-dihydropyridine-3-carboxylic acid as a brown solid (0.740 g, 79% yield). The melting point of the product was 256-263°C. The structure of the product was determined by 1H NMR (400 MHz, DMSO-d6) δ 6.53 (d, J = 9.4 Hz, 1H), 7.40-7.49 (m, 5H), 7.87 (dd, J = 2.5, 9.8 Hz, 1H), 8.23 (d, J = 2.5 Hz, 1H); IR (KBr) ν 3446, 1708, 1645, 1577, 1263, 1228 cm-1; MS 214 (M-1) for characterization.
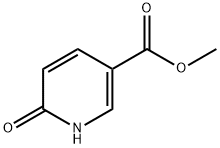
66171-50-4
169 suppliers
$12.00/1 / G

77837-08-2
60 suppliers
$92.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: pyridine; copper(II) acetate monohydrate / dichloromethane / 12 h / 20 °C / Molecular sieve
2.1: water; lithium hydroxide monohydrate / tetrahydrofuran / 1 h / 0 °C
2.2: pH 2
References:
AUSPEX PHARMACEUTICALS, INC.;ZHANG, Chengzhi;SOMMERS, Andreas WO2012/122165, 2012, A2

77837-09-3
43 suppliers
$120.00/10mg

77837-08-2
60 suppliers
$92.00/100mg

62-53-3
691 suppliers
$10.00/1g

77837-08-2
60 suppliers
$92.00/100mg
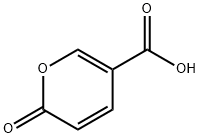
500-05-0
260 suppliers
$26.00/5g

77837-08-2
60 suppliers
$92.00/100mg
5006-66-6
485 suppliers
$13.43/1gm:

77837-08-2
60 suppliers
$92.00/100mg