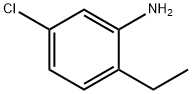
5-chloro-2-ethylbenzenamine synthesis
- Product Name:5-chloro-2-ethylbenzenamine
- CAS Number:3843-97-8
- Molecular formula:C8H10ClN
- Molecular Weight:155.62
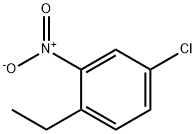
2001-16-3

3843-97-8
Step 3: Synthesis of 5-chloro-2-ethylaniline A methanol (50 mL) solution of hydrazine hydrate (6.95 mL, 134.7 mmol) was slowly added dropwise to a methanol (120 mL) solution of 4-chloro-1-ethyl-2-nitrobenzene (6.25 g, 33.7 mmol). Ferric (III) chloride (547 mg, 3.4 mmol) and activated carbon (547 mg) were added as catalysts to the reaction system. The reaction mixture was stirred under reflux conditions for 13 hours. After completion of the reaction, the solid catalyst was removed by diatomaceous earth filtration and the filtrate was concentrated. The resulting crude product was purified by fast chromatography (eluent: hexane/ethyl acetate = 9:1) to afford the light pink oily target compound 5-chloro-2-ethylaniline (5.09 g, 97% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.09 (t, J = 7.51 Hz, 3H), 2.39 (q, J = 7.49 Hz, 2H), 5.13 (s, 2H), 6.47 (dd, J = 8.06, 2.20 Hz, 1H), 6.62 (d, J = 2.20 Hz, 1H), 6.89 (d, J = 8.06 Hz, 1H). J = 8.06 Hz, 1H).
116-69-8
95 suppliers
$38.00/250mg

3843-97-8
44 suppliers
$47.00/100mg
Yield:3843-97-8 100%
Reaction Conditions:
with iron(III) chloride;amino aminoalcohol in methanol at 20 - 80; for 5 h;Sealed tube;
Steps:
1.3
Step 3:ο a mixture of 4-chloro-l-ethyl-2-nitrobenzene (0.9 g, 4.9 mmol), iron (III) chloride (0.13 g, 0.49 mmol), and charcoal (80 mg, 6.6 mmol) in methanol (17 ml) was added hydrazine hydrate (0.95 ml, 20 mmol) in methanol (7 ml). The reaction mixture was stirred at rt as gas was evolved. When gas evolution ceased, the vial was sealed and heated at 80 deg C for 5 h (**pressure builds over time and vial needed to be vented frequently). The mixture was cooled to rt and filtered through celite washing with methanol, concentrated, and purified by silica gel chromatography (gradient 0 to 50% EtOAc/hexanes). The product, aniline 4, is a light yellow oil (quant.); 1H NMR (400 MHz, DMSO-d6) δ 6.86 (dd, = 8.0, 0.7 Hz, 1H), 6.59 (d, = 2.2 Hz, 1H), 6.44 (dd, 7 = 8.0, 2.2 Hz, 1H), 5.11 (s, 2H), 2.43 - 2.31 (m, 2H), 1.06 (t, J = 7.5 Hz, 3H).
References:
WO2016/106331,2016,A1 Location in patent:Paragraph 0222
589-16-2
224 suppliers
$10.00/1g

3843-97-8
44 suppliers
$47.00/100mg
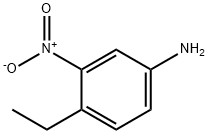
51529-96-5
37 suppliers
inquiry

3843-97-8
44 suppliers
$47.00/100mg
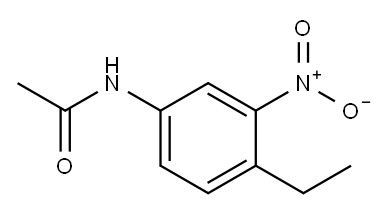
5805-89-0
1 suppliers
inquiry

3843-97-8
44 suppliers
$47.00/100mg

3663-34-1
28 suppliers
$106.00/50mg

3843-97-8
44 suppliers
$47.00/100mg