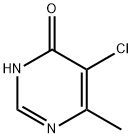
5-CHLORO-6-METHYLPYRIMIDIN-4(1H)-ONE synthesis
- Product Name:5-CHLORO-6-METHYLPYRIMIDIN-4(1H)-ONE
- CAS Number:7752-72-9
- Molecular formula:C5H5ClN2O
- Molecular Weight:144.56
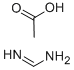
3473-63-0
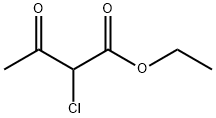
609-15-4

7752-72-9
The general procedure for the synthesis of 5-chloro-6-methylpyrimidin-4(1H)-one from formamidine acetate and ethyl 2-chloroacetoacetate was as follows: a methanolic solution of 4-hydroxy-5-chloro-6-methylpyrimidine (8.80 g, 0.16 mol) was slowly added to a 50 mL methanolic solution containing 11.30 g (0.11 mol) of formamidine acetate, and the reaction was carried out with stirring at room temperature. After the addition was completed, the mixture continued to be stirred at room temperature for 2 hours. Subsequently, 11.17 g (0.068 mol) of ethyl 2-chloro-3-oxobutanoate was added and the reaction continued to be stirred for 5-7 hours at room temperature. The reaction process was monitored by thin layer chromatography (TLC). After completion of the reaction, the reaction mixture was concentrated under reduced pressure, the pH was adjusted to 5-6 with hydrochloric acid (HCl) and filtered to give an orange-yellow solid. The aqueous phase was extracted with ethyl acetate (3 x 50 mL), the organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was dissolved in 50 mL of ethyl acetate and allowed to stand overnight to give 6.48 g of orange-yellow solid product in 66% yield with a melting point of 181-184°C.

3473-63-0
520 suppliers
$5.00/25g

609-15-4
461 suppliers
$6.00/25g

7752-72-9
51 suppliers
$40.00/100mg
Yield:7752-72-9 66%
Reaction Conditions:
Stage #1: formamidine acetic acidwith sodium methylate in methanol at 20; for 2 h;
Stage #2: ethyl 2-chloro-3-oxo-butyrate in methanol at 20;
Steps:
1.1 preparation of 4-hydroxyl-5-chloro-6-methylpyrimidine
The preparation of 4-hydroxyl-5-chloro-6-methylpyrimidine ;8.80g (0.16mol) of CH3ONa in methanol was added slowly to a solution of 11.30g (0.11mol of formimidamide in 50 mL of methanol at room temperature under stirring, the mixture was stirred for another 2 hrs after addition at room temperature. ;Followed by addition of 11.17g ( 0.068mol ) of ethyl 2-chloro-3-oxobutanoate, the mixture was continued stirring for another 5-7 hrs at room temperature. ;After the reaction was over by Thin-Layer Chromatography monitoring, the reaction mixture was concentrated under reduced pressure and pH was adjusted to 5-6 with HCl, and then filtered to afford orange-yellow soilid, the water phase was extracted with ethyl acetate (3x50mL), dried over anhydrous magnesium sulfate, filtered and then concentrated under reduced pressure. ;The residue was dissolved to 50ml of ethyl acetate, stand overnight to obtain 6.48g as orange-yellow soilid with yield of 66%. m.p. 181~184°C.
References:
EP2913325,2015,A1 Location in patent:Paragraph 0451; 0452
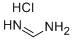
6313-33-3
204 suppliers
$9.00/1g

609-15-4
461 suppliers
$6.00/25g

7752-72-9
51 suppliers
$40.00/100mg
