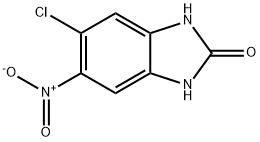
5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one synthesis
- Product Name:5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one
- CAS Number:60713-78-2
- Molecular formula:C7H4ClN3O3
- Molecular Weight:213.58
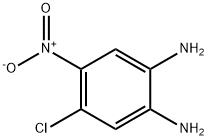
67073-39-6
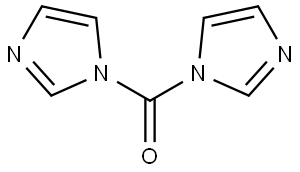
530-62-1
![5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one](/CAS/GIF/60713-78-2.gif)
60713-78-2
4-Chloro-5-nitro-1,2-benzenediamine (8.34 g, 44.4 mmol) was dissolved in tetrahydrofuran (625 mL) at 0 °C, followed by the addition of N,N'-carbonyl diimidazole (8.65 g, 53.3 mmol). The reaction mixture was slowly warmed to 23 °C and stirred continuously at this temperature for 20 hours. Upon completion of the reaction, the mixture was concentrated to about 300 mL, and then 1 M aqueous hydrochloric acid (500 mL) and water (total volume adjusted to 2 L) were added. The resulting suspension was cooled at 0 °C for 2 h. The precipitate was collected by filtration and dried on a filter. Finally, the precipitate was ground with cold ethyl acetate (20 mL) and rinsed with ethyl acetate (2 x 5 mL) to afford 5-chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one (7.26 g, 76% yield). Mass spectrometry (ESI/CI) analysis: calculated value C7H4ClN3O3, 213.0; measured value m/z, 214.0 [M + H]+.

67073-39-6
52 suppliers
$36.00/100mg

530-62-1
967 suppliers
$6.00/25g
![5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one](/CAS/GIF/60713-78-2.gif)
60713-78-2
25 suppliers
inquiry
Yield:60713-78-2 76%
Reaction Conditions:
in tetrahydrofuran at 0 - 23;
Steps:
40.A
To a solution of 4-chloro-5-nitro-benzene-1 ,2-diamine (8.34 g, 44.4 mmol) and THF (625 ml_) was added carbonyl diimidazole (8.65 g, 53.3 mmol) at 0 0C. The reaction mixture was allowed to warm to 23 0C and was stirred for 20 h at this temperature. The reaction mixture was concentrated to a volume of 300 ml_ and 500 ml_ aqueous 1 M HCI was added, followed by water (total volume 2 L). The resulting suspension was cooled at 0 0C for 2 h, and the precipitate was collected and dried on the filter. It was then triturated with cold EtOAc (20 ml_) and rinsed EtOAc (2 x 5 ml_) to yield the titled compound (7.26 g, 76% yield). MS (ESI/CI): mass calcd. for C7H4CIN3O3, 213.0; m/z found, 214.0 [M+H]+.
References:
WO2009/134750,2009,A1 Location in patent:Page/Page column 80

67073-39-6
52 suppliers
$36.00/100mg
![5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one](/CAS/GIF/60713-78-2.gif)
60713-78-2
25 suppliers
inquiry
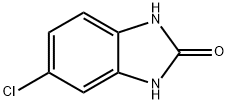
2034-23-3
79 suppliers
$9.00/100mg
![5-Chloro-6-nitro-1H-benzo[d]imidazol-2(3H)-one](/CAS/GIF/60713-78-2.gif)
60713-78-2
25 suppliers
inquiry