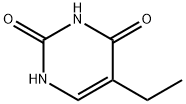
5-Ethyluracil synthesis
- Product Name:5-Ethyluracil
- CAS Number:4212-49-1
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14
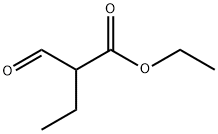
36873-42-4
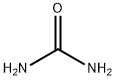
57-13-6

4212-49-1
Urea (19.39 g, 0.32 mol, JT Baker) was slowly added to fuming sulfuric acid (26-29.5% free SO3, 135 mL, 2.65 mol, Aldrich) under cooling in an ice-water bath over a period of 20 min, and the reaction temperature was controlled to be maintained at 8 to 15°C. After stirring for 30 min, ethyl 2-formylbutyrate was added dropwise over 18 min ( 46.55 g, 0.32 mol, from Example LC), keeping the reaction temperature constant. After continued stirring for 30 minutes, a second portion of urea (15.07 g, 0.25 mol) was added over 10 minutes at the same temperature. Subsequently, the reaction mixture was stirred at room temperature for 65 hours and then heated at 90-100°C for 2 hours (gas was observed to escape, the reaction was exothermic, and the temperature rose to 110°C). Upon completion of the reaction, the mixture was cooled to 30 °C using an ice water bath and ice (270 g) was slowly added to ensure that the temperature did not exceed 35 °C. The mixture was further cooled to 5 °C and stirred for 20 min. The precipitated solid was collected by filtration, washed sequentially with cold water, hexane and ether and dried by filtration to give 5-ethyluracil in 38.85 g (85.9%) yield.
Yield: 85.9%
Reaction Conditions:
with sulfuric acid;sulfur trioxide at 8 - 100; for 66 h;
Steps:
1c 5-Ethyl uracil
Urea (19.39 g, 0.32 mol) (J. T. Baker) was added over 20 minutes to fuming sulfuric acid (26-29.5% free S03,135 mL, 2.65 mol) (Aldrich) with cooling in an ice water bath maintaining the reaction temperature between 8 to 15 °C. After stirring for an additional 30 minutes, ethyl 2-formylbutyrate (46.55 g, 0.32 mol) (from Example LC, supra) was added over 18 minutes keeping the reaction at the same temperature. After stirring for another 30 minutes, a second portion of urea (15.07 g, 0.25 mol) was added over 10 minutes at the same temperature. The reaction mixture was then stirred at room temperature for 65 hours, and at 90-100 °C for 2 hours (gas evolution was observed, and reaction was exothermic, with reaction temperature rising to 110 °C). The mixture was cooled to 30 °C with an ice-water bath. Ice (270 g) was added slowly keeping the reaction below 35 °C. The mixture was then cooled to 5 °C and stirred for 20 minutes. The solid formed was collected by filtration, washed with cold water, hexanes, and diethyl ether and dried by suction to give 5-ethyl uracil. (Yield 38.85 g, 85.9%).
References:
F. HOFFMANN-LA ROCHE AG WO2004/41821, 2004, A1 Location in patent:Page 26
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4212-49-1
190 suppliers
$5.00/1g
34171-37-4
62 suppliers
$29.00/250mg

4212-49-1
190 suppliers
$5.00/1g
25198-98-5
44 suppliers
$45.00/10mg
2518-72-1
29 suppliers
inquiry

4212-49-1
190 suppliers
$5.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4212-49-1
190 suppliers
$5.00/1g