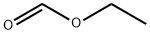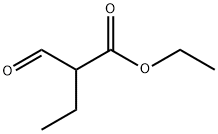
ethyl 2-formylbutanoate synthesis
- Product Name:ethyl 2-formylbutanoate
- CAS Number:36873-42-4
- Molecular formula:C7H12O3
- Molecular Weight:144.17
Yield:36873-42-4 55.1%
Reaction Conditions:
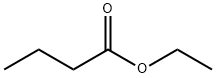
Stage #1: Ethyl butyratewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexanes at -75 - 0; for 0.966667 h;
Stage #2: formic acid ethyl ester in tetrahydrofuran;hexanes at -75 - 20; for 3.41667 h;
Steps:
1c Ethyl 2-formylbutyrate
A solution of diisopropylamine (120.6 mL, 0.86 mol) (Aldrich) in tetrahyrofuran (370 mL) was cooled TO-30 °C. n-Butyllithium (2.5 M in hexanes, 344.2 mL, 0.86 mol) (Aldrich) was added drop wise at such a rate that the reaction mixture temperature was kept between-30 to 0 °C. The reaction mixture was then cooled to-75 °C in a dry ice- acetone bath. A solution of ethyl butyrate (100 g, 0.86 mol) (Aldrich) in tetrahydrofuran (170 mL) was added drop wise over 28 minutes and keeping the reaction temperature between-75 TO-70 °C. The mixture was stirred at the same temperature for an additional 30 minutes. Ethyl formate (125 mL, 1.55 mol) (Aldrich) was then added to this mixture over 25 minutes and maintaining the temperature between-75 TO-70 °C. The resultant mixture was allowed to warm to room temperature and stirred at room temperature for 3 hours. With external cooling in a cold water bath to keep the reaction temperature below 30 °C acetic acid (98.55 mL, 1.72 mol) was added, followed by water (430 mL) and dichloromethane (200 mL). After separating the layers, the organic layer was washed with water (300 mL). The combined water layer was extracted with dichloromethane (200 mL). The combined organic layer was washed with aqueous sodium bicarbonate solution (200 mL). The basic aqueous solution was extracted with dichloromethane (100 mL). All organic layers were then combined, dried over sodium sulfate over night, filtered and distilled to remove solvent leaving about 180 mL. (Some of the product was distilled over with tetrahydrofuran. ) The residue was distilled at 65- 81 °C (23 mm Hg). The fraction distilling over at 70-81 °C (23 mm Hg) gave ethyl 2- formylbutyrate. (Yield 68. 35 G, 55.1%).
References:
WO2004/41821,2004,A1 Location in patent:Page 25-26

99437-71-5
4 suppliers
inquiry

36873-42-4
2 suppliers
inquiry
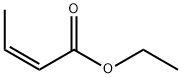
6776-19-8
4 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

36873-42-4
2 suppliers
inquiry

54998-57-1
3 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
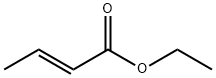
623-70-1
177 suppliers
$10.00/5g

36873-42-4
2 suppliers
inquiry

54998-57-1
3 suppliers
inquiry