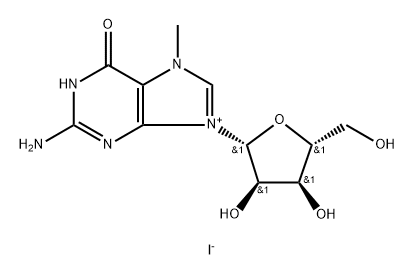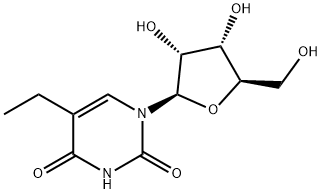
5-Ethyluridine synthesis
- Product Name:5-Ethyluridine
- CAS Number:25110-76-3
- Molecular formula:C11H16N2O6
- Molecular Weight:272.25
Yield:25110-76-3 67%
Reaction Conditions:
with potassium dihydrogenphosphate;E. coli purine nucleoside phosphorylase;E. coli uridine phosphorylase in aq. buffer at 20; pH=7.5; for 20 h;Enzymatic reaction;
Steps:
2.3.2. Ribothymidine (2b)
General procedure: To a solution of Thy (37 mg, 0.3 mmol), 7-MeGuo hydroiodide (191 mg, 0.45 mmol) and potassium dihydrophosphate (KH2PO4, 20 mg, 0.15 mmol) in 50mM Tris-HCl buffer, pH 7.5, 80 mL) at ambient temperature, 5 μL of 32 mg/mL E. coli purine nucleoside phosphorylase (PNP) solution (1.48 U) and 12.5 μL E. coli uridine phosphorylase (UP) (8.60 U) were added in one portion. The reaction mixture was allowed to stand for 5 h at ambient temperature under neat stirring and then allowed to stand for 15 h at ambient temperature without stirring. The conversion of the initial 7-MeGuo was controlled by HPLC. The reaction mixture was cooled to 0 °C and then filtered through nitrocellulose membrane Whatman (0.2 μm, 25 mm) to remove the white precipitate of 7-methylguanine (7-MeGua). The precipitate was washed with milli-Q water (15 mL). The combined transparent filtrate was concentrated under reduced pressure using a rotary evaporator to ca. 2 mL (bath temperature (5 mL) was then added to the suspension. The resulting mixture was concentrated to near dryness and co-evaporated with ethanol (2×20 mL). The dry residue was applied on a chromatographic column (diameter 20 mm) with silica gel (20 mL) for purification. For the protection, the silica gel layer was topped with ca 0.5-cm layer of sand. The column was washed with dichloromethane (25 mL), a mixture of dichloromethane and ethanol (95:5, v/v, 50 mL), and a mixture of dichloromethane and ethanol (90:10, v/v, 100 mL). The product was eluted with dichloromethane: ethanol (80:20, v/v, 100 mL) and 10 mL fractions were collected and evaporated in vacuo to dryness. The residue was co-evaporated 5 times with dichloromethane and dried using a vacuum pump at r.t. for 1 h.
References:
Alexeev, Cyril S.;Drenichev, Mikhail S.;Dorinova, Evgeniya O.;Esipov, Roman S.;Kulikova, Irina V.;Mikhailov, Sergey N. [Biochimica et Biophysica Acta - Proteins and Proteomics,2020,vol. 1868,# 1,art. no. 140292]
25692-02-8
9 suppliers
inquiry

25110-76-3
18 suppliers
inquiry
4212-49-1
195 suppliers
$5.00/1g

25110-76-3
18 suppliers
inquiry
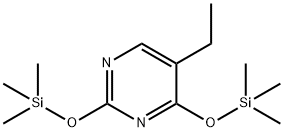
31167-05-2
3 suppliers
inquiry

25110-76-3
18 suppliers
inquiry
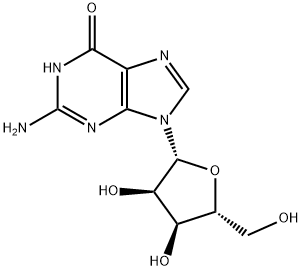
118-00-3
646 suppliers
$5.00/10g

25110-76-3
18 suppliers
inquiry