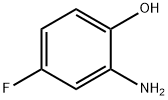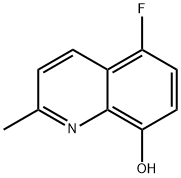
5-Fluoro-2-methyl-quinolin-8-ol synthesis
- Product Name:5-Fluoro-2-methyl-quinolin-8-ol
- CAS Number:37026-20-3
- Molecular formula:C10H8FNO
- Molecular Weight:177.18
Yield:37026-20-3 84%
Reaction Conditions:
with hydrogenchloride in water;toluene; for 3 h;Reflux;
Steps:
4 Synthesis of 5-fluoro-2-methylquinolin-8-ol (1c)
Toluene (30 mL) and crotonaldehyde (2.6 mL, 31 mmol) were added to a solution of 2-amino-4-fluorophenol (2.g, 15.7 mmol) in aqueous 6 M HCl (200 mL), and were heated under reflux for 3 h. The mixture was allowed to cool to room temperature.
The aqueous layer was separated and neutralized with aqueous solution of K2CO3.
After extraction with CH2Cl2 (3 * 50 mL), the organic layer was separated and dried over MgSO4, then was filtered and evaporated. The residue was purified by chromatography and crystallization. 5-Fluoro-2-methylquinolin-8-ol (1c) 2.33 g (13.2 mmol, 84%), mp = 57-58 °C (lit. 57-58 °C
[15]
); 1H NMR (DMSO-d6; 400.2 MHz) δ = 2.72 (s, 3H, CH3), 7.00 (dd, JHH = 8.5 Hz, JHF = 5.0 Hz, 1H, H-quinoline), 7.18 (m, 1H, H-quinoline), 7.56 (d, JHH = 8.6 Hz, 1H, H-quinoline), 8.30 (d, JHH = 8.6 Hz, 1H, H-quinoline), 9.45 (bs, 1H, H-quinoline); 1H NMR (CDCl3; 400.2 MHz) δ = 2.74 (s, 3H, CH3), 7.02 (dd, JHH = 8.5 Hz, JHF = 2.8 Hz, 1H, H-quinoline), 7.04 (dd, JHH = 8.5 Hz, JHF = 6.8 Hz, 1H, H-quinoline), 7.36 (d, JHH = 8.6 Hz, 1H, H-quinoline), 8.26 (d, JHH = 8.6 Hz, 1H, H-quinoline), 9.45 (bs, 1H, H-quinoline); 13C{1H} NMR (DMSO-d6; 100.6 MHz) δ = 24.71, 109.58 (d, JCF = 20.4 Hz), 109.78 (d, JCF = 8.1 Hz), 116.64 (d, JCF = 18.0 Hz), 122.84 (d, JCF = 2.6 Hz), 128.95 (d, JCF = 3.1 Hz), 137.54 (d, JCF = 3.1 Hz), 149.06 (d, JCF = 3.1 Hz), 149.68 (d, JCF = 242.8 Hz), 157.84; 19F{1H} NMR (DMSO-d6; 188.3 MHz) δ = -134.97; 19F NMR (DMSO-d6; 188.3 MHz) δ = -134.38 (dd, JFH = 10.0 Hz, JFH = 5.0 Hz); MS: (ESI) (M+H)+ = 178.
References:
Nycz, Jacek E.;Malecki, Grzegorz J. [Journal of Molecular Structure,2013,vol. 1032,p. 159 - 168]