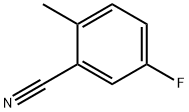
5-Fluoro-2-methylbenzonitrile synthesis
- Product Name:5-Fluoro-2-methylbenzonitrile
- CAS Number:77532-79-7
- Molecular formula:C8H6FN
- Molecular Weight:135.14
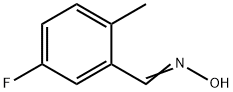
953422-44-1

77532-79-7
The general procedure for the synthesis of 5-fluoro-2-methylbenzonitrile from the compound (CAS: 953422-44-1) was as follows: aldoxime (1 mmol) and imidazole (3 mmol) were dissolved in anhydrous dichloromethane (10-15 mL) under nitrogen protection, added to a pre-oven-dried round-bottomed flask, and the reaction mixture was stirred at room temperature.After 20 min, the reaction mixture was stirred via syringe under After 20 min, trifluoromethanesulfonic anhydride (0.4-1.0 mmol) was slowly added dropwise via syringe under nitrogen protection and the reaction mixture was continued to be stirred at room temperature for a specific time (see Table 1). The reaction process was monitored by thin layer chromatography (TLC) and/or gas chromatography-mass spectrometry (GC-MS). Upon completion of the reaction, the reaction was quenched with dilute sodium bicarbonate (NaHCO3) solution and the product was extracted with dichloromethane. The solvent was removed by concentration under reduced pressure to give the crude product, which was subsequently purified by preparative thin-layer chromatography (TLC) using a solvent mixture of ethyl acetate-hexane (80:20) to give the target nitrile analogs in 61-88% yield. The structure of the products was confirmed by melting point (mp) or boiling point (bp), TLC, infrared spectroscopy (IR), GC-MS, 1H nuclear magnetic resonance (NMR), and 13C NMR data in comparison with authentic samples commercially purchased or prepared by literature methods.

953422-44-1
1 suppliers
inquiry

77532-79-7
197 suppliers
$10.00/1g
Yield: 82%
Reaction Conditions:
with trifluoromethylsulfonic anhydride in dichloromethane at 20; for 6 h;Inert atmosphere;
Steps:
General procedure for synthesis of nitriles from oximes (1-16):
General procedure: A mixture of aldoximes (1 mmol) and imidazole (3 mmol) in anhydrous DCM (10-15 mL) was charged into an oven-dried round-bottom flask under nitrogen and the reaction mixture was stirred at r.t. for 20 min, after which triflic anhydride (0.4-1.0 mmol) was added dropwise via syringe under nitrogen, and the reaction mixture was allowed to stir at r.t. for a specific time (Table 1). Progress of the reaction was monitored by TLC and/or by GC-MS. Upon completion, the reaction mixture was quenched with dilute NaHCO3 solution and the product mass was extracted in DCM. The solvent was removed under reduced pressure to yield the crude product which was purified by prep TLC (ethyl acetate-hexane mixture) (80:20), to give the corresponding nitrile (61-88% yield). The structure of the products was confirmed by comparison of their mp or bp, TLC, IR, GC-MS, 1H NMR and 13C NMR data with authentic samples obtained commercially or prepared by literature methods.
References:
Kalkhambkar, Rajesh G.;Bunge, Scott D.;Laali, Kenneth K. [Tetrahedron Letters,2011,vol. 52,# 40,p. 5184 - 5187] Location in patent:supporting information; experimental part
1422-53-3
339 suppliers
$5.00/5g
55305-43-6
76 suppliers
$23.00/100mg

77532-79-7
197 suppliers
$10.00/1g
452-73-3
364 suppliers
$6.00/25g

77532-79-7
197 suppliers
$10.00/1g