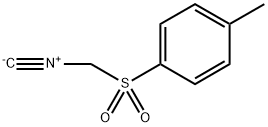
Tosylmethyl isocyanide synthesis
- Product Name:Tosylmethyl isocyanide
- CAS Number:36635-61-7
- Molecular formula:C9H9NO2S
- Molecular Weight:195.24
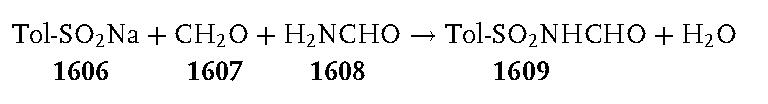
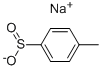
A 3-L, three-necked, round-bottomed flask, equipped with a mechanical stirrer, a condenser, and a thermometer, was charged with sodium p-toluenesulfinate 1606 (267 g, 1.5 mol). After the addition of water (750 mL), a 34–37% solution of formaldehyde 1607 (350 mL, 378 g, ca. 4.4 mol), formamide 1608 (600 mL, 680 g, 15 mol), and formic acid (200 mL, 244 g, 5.3 mol), the stirred reaction mixture was heated at 90 C°. The sodium p-toluenesulfinate dissolved during the heating, and the clear solution was kept at 90–95 C° for 2 h. It was then cooled in an ice/salt bath with continued stirring and further cooled overnight in a freezer at 20 C°. The white solid produced was collected by suction filtration. It was washed thoroughly in a beaker by stirring with three 250 mL portions of iced water. The product was dried under reduced pressure over phosphorus pentoxide at 70 C° to provide 134–150 g (42–47%) of crude N-(p-tolylsulfonylmethyl)formamide 1609; mp 106–110 C°. This product was sufficiently pure to be used directly in the following reaction.
36635-56-0
90 suppliers
inquiry

36635-61-7
522 suppliers
$5.00/5g
Yield:36635-61-7 80%
Reaction Conditions:
with triethylamine;trichlorophosphate in dichloromethane at -3 - 0; for 1 h;
Steps:
1
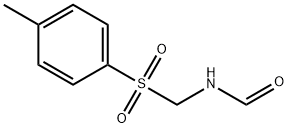
Add 42.6g of p-toluenesulfonylmethylformamide, 300mL of dichloromethane, 112mL of triethylamine, and ice salt to a four-necked flask equipped with a thermometer, a stirrer, a reflux condenser with a gas guide tube and a dropping funnel. Cool the bath to -3°C, add dropwise a mixed solution of 20mL of phosphorus oxychloride and 20mL of dichloromethane, control the temperature below 0°C, stir for 1 hour, add 300mL of sodium hydroxide solution with a mass concentration of 7%, and stir for 0.5 After hours of liquid separation, the organic layer was washed once with water and dried with anhydrous sodium sulfate. The filtrate obtained by filtration was first normal pressure and then dichloromethane was evaporated under reduced pressure until solids appeared on the bottle wall, and then 50 mL petroleum ether was added to the distillation flask , Stand for 1 hour under cooling with ice water, the precipitated crystals are suction filtered, dried, and recrystallized with petroleum ether to obtain 31.2 g of light brown p-toluenesulfonyl methyl isocyanide with a purity of 98% and a yield of 80%.
References:
CN111393348,2020,A Location in patent:Paragraph 0143; 0152; 155
824-79-3
420 suppliers
$6.00/25g

36635-61-7
522 suppliers
$5.00/5g
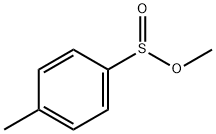
672-78-6
31 suppliers
inquiry

36635-61-7
522 suppliers
$5.00/5g
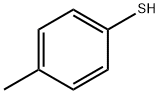
106-45-6
231 suppliers
$10.00/1g

36635-61-7
522 suppliers
$5.00/5g
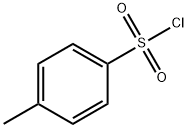
98-59-9
625 suppliers
$9.00/5g

36635-61-7
522 suppliers
$5.00/5g