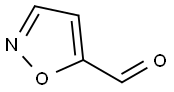
5-FORMYLISOXAZOLE synthesis
- Product Name:5-FORMYLISOXAZOLE
- CAS Number:16401-14-2
- Molecular formula:C4H3NO2
- Molecular Weight:97.07
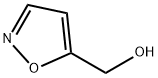
98019-60-4

16401-14-2
Step 2: Synthesis of isoxazole-5-carbaldehyde In a round bottom flask, isoxazol-5-yl-methanol (511 mg, 5.16 mmol) was dissolved in dichloromethane (30 mL). Dess-Martin periodate (2.3 g, 5.41 mmol) was added and the reaction mixture was stirred for 1.5 h at room temperature. Upon termination of the reaction, 50 mL of 10% aqueous Na2S2O3 and saturated aqueous NaHCO3 were added, followed by extraction with dichloromethane (2 x 30 mL). The organic layer was washed sequentially with saturated NaHCO3 aqueous solution, water and brine. The aqueous layer was back-extracted with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography using EtOAc/hexane (gradient 0-40% EtOAc) as eluent to afford 226 mg (45% yield) of isoxazole-5-carbaldehyde as a colorless oil.1H NMR (CDCl3, 300 MHz) δ (ppm): 10.05 (s, 1H), 8.44 (d, J = 1.9 Hz, 1H). 7.02 (d, J = 1.9 Hz, 1H).

98019-60-4
77 suppliers
$93.00/100mg

16401-14-2
52 suppliers
$217.50/250mg
Yield: 45%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 1.5 h;
Steps:
55.2 Step 2Isoxazole-5-carbaldehyde
Step 2Isoxazole-5-carbaldehydeIn a round-bottomed flask, isoxazol-5-yl-methanol (511 mg, 5.16 mmol) was dissolved in dichloromethane (30 ml). Dess-Martin periodinane (2.3 g, 5.41 mmol) was added and the reaction mixture was stirred at room temperature for 1.5 h. The reaction was quenched with 50 ml of a 1 :1 solution of 10%> aqueous Na2S203 and saturated aqueous NaHCC>3 and then extracted with dichloromethane (2x). The organic layers were washed with saturated aqueous NaHCC>3, water and brine. The aqueous layers were back extracted with dichloromethane. The organic layers were combined, dried over sodium sulfate, filtered and concentrated. The residue was chromato graphed over silica gel with EtOAc/hexanes (gradient 0-40% EtOAc) to afford 226 mg (45%) of isoxazole-5-carbaldehyde as a colorless oil. 1H NMR (CDC13, 300 MHz): ? (ppm)10.05 (s, 1H), 8.44 (d, J=1.9 Hz, 1H), 7.02 (d, J=1.9 Hz, 1H).
References:
F. HOFFMANN-LA ROCHE AG;CHEN, Shaoqing;DE VICENTE FIDALGO, Javier;HAMILTON, Matthew Michael;HERMANN, Johannes Cornelius;KENNEDY-SMITH, Joshua;LI, Hongju;LOVEY, Allen John;LUCAS, Matthew C.;LUK, Kin-Chun Thomas;LYNCH, Stephen M.;O'YANG, Counde;PADILLA, Fernando;SCHOENFELD, Ryan Craig;SIDDURI, Achyutharao;SOTH, Michael;WANG, Ce;WOVKULICH, Peter Michael;ZHANG, Xiaohu WO2013/30138, 2013, A1 Location in patent:Page/Page column 154
15055-81-9
95 suppliers
$104.00/1 g

16401-14-2
52 suppliers
$217.50/250mg