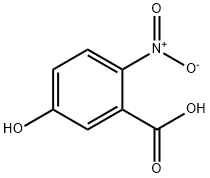
5-Hydroxy-2-nitrobenzoic acid synthesis
- Product Name:5-Hydroxy-2-nitrobenzoic acid
- CAS Number:610-37-7
- Molecular formula:C7H5NO5
- Molecular Weight:183.12
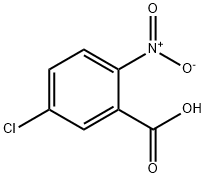
2516-95-2

610-37-7
General procedure for the synthesis of 2-nitro-5-hydroxybenzoic acid from 5-chloro-2-nitrobenzoic acid: 5-chloro-2-nitrobenzoic acid (32.00 g, 0.159 mol) was dissolved in 15% aqueous sodium hydroxide solution and reacted at reflux under nitrogen protection for 72 hours. After completion of the reaction, the pH of the reaction solution was adjusted to 1.0 with dilute hydrochloric acid and then extracted with ethyl acetate. The ethyl acetate extracts were combined and dried with anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure to give 28.05 g of 2-nitro-5-hydroxybenzoic acid in 96.5% yield.
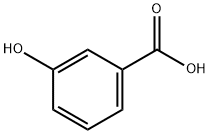
99-06-9
528 suppliers
$5.00/25g

610-37-7
170 suppliers
$16.00/1g
Yield: 70%
Reaction Conditions:
with copper(II) nitrate trihydrate in tetrahydrofuran for 3 h;Reflux;regioselective reaction;
Steps:
General experimental procedure for nitration of phenols
General procedure: A suspension of 2-methylphenol(18.5 mmol, 1.0 eq) and Cu(NO3)2.3H2O (27.7 mmol, 1.5 eq) in THF was stirred magnetically at 60°C or reflux for several hours. Then after the solvent was removed under vacuum, the mixture was extracted with EtOAc (3×30 mL). The combined organic layers were washed with brine (5mL), dried over anhydrous MgSO4 and concentrated under vacuum. The crude residue was purified by column chromatography to afford the product (67-90%).
References:
Chen, Ling-Yan;Liu, Tao;Zhou, Xiaokun;Sun, Zhihua [ARKIVOC,2014,vol. 2014,# 5,p. 64 - 71]

2516-95-2
323 suppliers
$5.00/5g

610-37-7
170 suppliers
$16.00/1g
42454-06-8
215 suppliers
$8.00/1g

610-37-7
170 suppliers
$16.00/1g

99-06-9
528 suppliers
$5.00/25g

610-37-7
170 suppliers
$16.00/1g
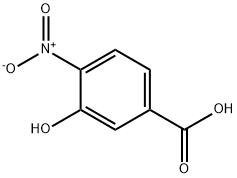
619-14-7
303 suppliers
$10.00/5g
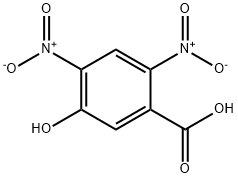
54797-73-8
1 suppliers
inquiry
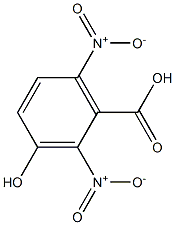
67175-28-4
1 suppliers
inquiry
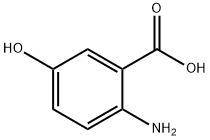
394-31-0
282 suppliers
$7.00/5g

610-37-7
170 suppliers
$16.00/1g