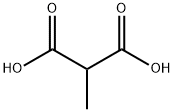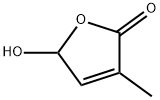
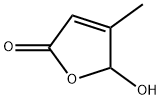
5-hydroxy-3-Methyl-2(5H)-furanone synthesis
- Product Name:5-hydroxy-3-Methyl-2(5H)-furanone
- CAS Number:931-23-7
- Molecular formula:C5H6O3
- Molecular Weight:114.1
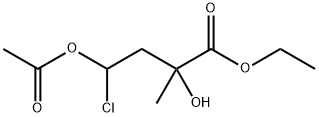
80350-42-1
8 suppliers
inquiry

931-23-7
48 suppliers
$97.00/200MG
Yield:931-23-7 65%
Reaction Conditions:
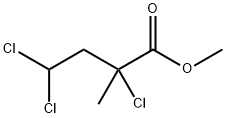
Stage #1:ethyl 4-acetoxy-4-chloro-2-hydroxy-2-methylbutanoate with hydrogenchloride;acetic acid in ethanol for 4 h;Reflux;
Stage #2: with water in ethanol for 0.75 h;Reflux;
Steps:
8 Hydrolysis and cyclization
[0425j A 1000 mL round bottom flask containing the crude aldol product (30.55 g, 128 mmol) was equipped with an oversized stirbar and taken up in absolute ethanol (345 mL) to give a yellow solution. To the stirred solution was added glacial acetic acid (17 mL) and concentrated HC1 (17 mL). A refiux condenser was fitted to the flask and the solution heated to reflux for 4 hours. At this time, deionized water (430 mL) was added and the ethanol removed by fractional distillation until distillation rate slows and internal temperature rises to approximately 90°C and volume of distillate is approximately 135% of initially added ethanol. The condenser was returned to reflux set up and the deep golden reaction mixture heated at reflux for 45 minutes. The cooled reaction mixture was extracted with ethyl acetate (3x 150 mL). Combined extracts were washed with brine (lx 100 mL) and dried Na2SO4. Filtered to give a golden solution, concentrated to give an orange oil (15.19 g). The crude orange oil was subjected to bulb-to-bulb distillation, collecting material at 120-135°C/8 mbar. The pale yellow oil (9.47 g, 65%) slowly solidified on standing.
References:
ASILOMAR BIO, INC.;DAVIDSON, Eric, A.;BAYER, Travis, S.;WINDRAM, Oliver;HLEBA, Yonek WO2015/61764, 2015, A1 Location in patent:Paragraph 0424; 0425
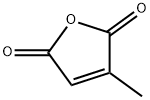
616-02-4
273 suppliers
$7.00/5g

931-23-7
48 suppliers
$97.00/200MG
40834-42-2
168 suppliers
$27.00/1g
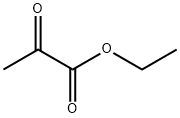
617-35-6
561 suppliers
$5.00/10g
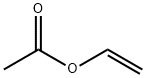
108-05-4
591 suppliers
$4.00/1000g

931-23-7
48 suppliers
$97.00/200MG
26459-55-2
0 suppliers
inquiry

931-23-7
48 suppliers
$97.00/200MG