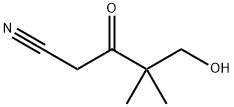
5-HYDROXY-4,4-DIMETHYL-3-OXOPENTANENITRILE synthesis
- Product Name:5-HYDROXY-4,4-DIMETHYL-3-OXOPENTANENITRILE
- CAS Number:489432-33-9
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
Yield:489432-33-9 29%
Reaction Conditions:
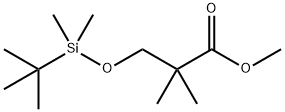
Stage #1: methyl 3-(tert-butyldimethylsilyloxy)-2,2-dimethyl-propanoate;acetonitrilewith sodium hydride in toluene;mineral oil; for 5 h;Reflux;
Stage #2: with hydrogenchloride in water;toluene at 20; pH=4;
Steps:
4.E.2
To a refluxing suspension of sodium hydride (1.40 g of a 60% suspension in mineral oil, 35 mmol) in anhydrous toluene (45 mL), was added dropwise (over 1 h) a mixture of methyl 3-(tert-butyldimethylsilyloxy)-2,2- dimethylpropanoate (6.0 g, 24.39 mmol) from Step A and acetonitrile (1.80 mL, 35 mmol). After refluxing for a further 5 h, the mixture was cooled to rt and the pH was adjusted to 4 with aqueous HCl solution. The mixture was extracted with ethyl acetate (3 x 200 mL) and the combined organic layers washed with brine (2 x 200 mL), separated, dried over Na2SO4, and filtered. Concentration under reduced pressure, followed by Purification via silica gel chromatography eluting with 33% ethyl acetate in petroleum ether, afforded 5-hydroxy-4,4-dimethyl-3-oxopentanenitrile as a yellow oil (1 g, 29%). 1H NMR (300 MHz, CDCl3) δ 3.76 (s, 2H), 3.61 (s, 2H), 1.19 (s, 6H).
References:
WO2010/54058,2010,A1 Location in patent:Page/Page column 80
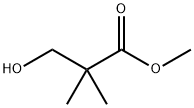
14002-80-3
182 suppliers
$7.00/5g

489432-33-9
12 suppliers
inquiry
14002-73-4
70 suppliers
$10.00/250mg

75-05-8
1049 suppliers
$10.00/10g

489432-33-9
12 suppliers
inquiry