
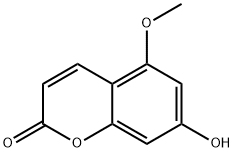
5-hydroxy-7-methoxy-chromen-2-one synthesis
- Product Name:5-hydroxy-7-methoxy-chromen-2-one
- CAS Number:23053-61-4
- Molecular formula:C10H8O4
- Molecular Weight:192.17
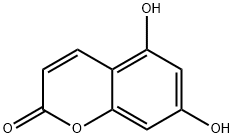
2732-18-5
106 suppliers
$16.00/100mg

74-88-4
357 suppliers
$15.00/10g

23053-61-4
3 suppliers
inquiry
Yield:23053-61-4 181.1 mg
Reaction Conditions:
Stage #1: 5,7-dihydroxy-2H-chromen-2-onewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 20; for 12 h;
Steps:
Synthesis of 5-hydroxy-7-methoxycoumarin (1b)
A solution of 5,7-dihydroxycoumarin (1a, 1.0 g,5.62 mmol) in DMF (15.0 mL) was treated with NaH (60%dispersion in oil, 224.8 mg, 11.2 mmol), and the mixturewas stirred for 0.5 h at ambient temperature. Iodomethane (0.8 mg, 5.62 mmol) was added dropwise to the reactionmixture and stirred for 12 h at ambient temperature. Saturatedaqueous NH4Cl was added and the solution wasevaporated. The residue was extracted with EtOAc. Theorganic layer was purified by HPLC {mobile phase: H2O/MeCN (75:25, v/v) [YMC triart PFP (250 9 10 mm i.d.)]}to give 5-hydroxy-7-methoxycoumarin (1b, 181.1 mg).1b: White powder; IR (ATR): mmax 1690, 1611, 1361,1218, 773 cm-1; EIMS: m/z 192 [M]+; HREIMS: m/z192.0421 (Calcd for C10H8O4 [M]+: m/z 192.0423); 1HNMR (400 MHz, methanol-d4) δ 3.81 (s), 6.06 (d,J = 9.2), 6.26 (d, J = 2.4), 6.35 (d, J = 2.4), 8.08 (d,J = 9.2).
References:
Matsumoto, Takahiro;Takahashi, Kazuki;Kanayama, Sumire;Nakano, Yuka;Imai, Hiromi;Kibi, Masumi;Imahori, Daisuke;Hasei, Tomohiro;Watanabe, Tetsushi [Natural Medicines,2017,vol. 71,# 4,p. 735 - 744]
2174-64-3
142 suppliers
$32.86/250mg
623-47-2
352 suppliers
$10.00/1g
3067-10-5
7 suppliers
inquiry

23053-61-4
3 suppliers
inquiry
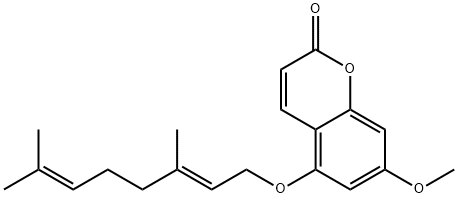
7380-39-4
49 suppliers
$75.00/1mg

23053-61-4
3 suppliers
inquiry

2174-64-3
142 suppliers
$32.86/250mg
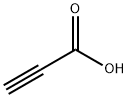
471-25-0
236 suppliers
$10.00/1g

3067-10-5
7 suppliers
inquiry

23053-61-4
3 suppliers
inquiry
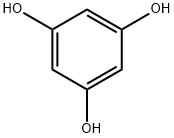
108-73-6
554 suppliers
$13.00/5g

23053-61-4
3 suppliers
inquiry