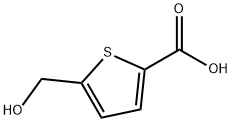
5-Hydroxymethyl-2-thiophene carbocylic acid synthesis
- Product Name:5-Hydroxymethyl-2-thiophene carbocylic acid
- CAS Number:14282-64-5
- Molecular formula:C6H6O3S
- Molecular Weight:158.18
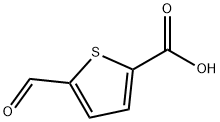
4565-31-5
142 suppliers
$19.00/250mg

14282-64-5
4 suppliers
inquiry
Yield:14282-64-5 100%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 20; for 8 h;Inert atmosphere;
Steps:
5.1.18. 5-(Hydroxymethyl)thiophene-2-carboxylic acid (17)
To a stirred solution of 5-formyl-2-thiophenecarboxylic acid 16 (5.0 g, 32.0 mmol) in MeOH (160 mL) was added NaBH4 (1.82 g, 48.0 mmol) at 0°C under nitrogen. The mixture was stirred at room temperature for 8 h. To the reaction mixture was added acetone and then evaporated. The residue was poured into 2 N HCl diluted with EtOAc. The aqueous layer was extracted with EtOAc. The combined organic extracts were washed with brine, dried over MgSO4, filtrated and then concentrated. The crude solid was washed with n-hexane/Et2O and filtrated to afford the title compound 17 as a light brown solid (quant.). 1H NMR (600 MHz, DMSO-d6): δ = 4.66 (s, 2H), 5.66 (br s, 1H), 6.99 (d, J = 3.8 Hz, 1H), 7.58 (d, J = 3.8 Hz, 1H), 12.90 (br s, 1H); 13C NMR (150 MHz, DMSO-d6): δ = 58.5, 124.3, 132.6, 133.0, 154.4, 163.0; HRMS-ESI m/z [M+H]+ calcd for C6H7O3S+:159.0110, found: 159.0112.
References:
Nagao, Satoshi;Yamane, Yoshinobu;Funasaka, Setsuo;Tanaka, Keigo;Miyazaki, Kazuki;Kotake, Yoshihiko;Kamata, Jun-Ichi;Watanabe-Miyano, Saori;Toyama, Osamu;Ozawa, Yoichi;Mizui, Yoshiharu;Okamoto, Kiyoshi;Ito, Daisuke [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 19,p. 5513 - 5529]
530145-16-5
1 suppliers
inquiry

14282-64-5
4 suppliers
inquiry