
5-Hydroxytetradecansaeure synthesis
- Product Name:5-Hydroxytetradecansaeure
- CAS Number:16899-03-9
- Molecular formula:C14H28O3
- Molecular Weight:244.37
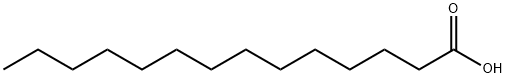
544-63-8
636 suppliers
$5.00/5g
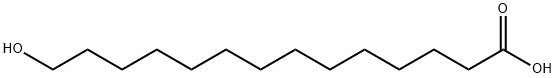
17278-74-9
41 suppliers
$85.00/50mg

16899-03-9
1 suppliers
inquiry
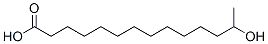
17278-73-8
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with 2,3,4,5,6-pentahydroxy-hexanal;cytochrome b5;glucose dehydrogenase from Bacillus megaterium;rabbit cytochrome P450 monooxygenase;rat cytochrome P450 reductase;1,2-dilauroyl-sn-glicero-3-phosphatidylcholine;NADPH;superoxide dismutase;catalase from bovine liver in aq. phosphate buffer;dimethyl sulfoxide at 30; pH=7.5;Enzymatic reaction;
Steps:
2.4 Fatty acid and fatty alcohol conversion by CYP4B1
General procedure: Conversions of fatty acids 1-8 and fatty alcohols 9-12 were carried out in 50mM potassium phosphate buffer, pH 7.5 and a total reaction volume of 100μL. Reaction mixtures contained 0.25μM CYP4B1, 0.5μM CPR, 0.25μM cytochrome b5, 100 U mL-1 superoxide dismutase, 1000 UmL-1 catalase, 25 U mL-1 GDH, 20mM glucose, 25μgmL-1 DLPC, 200μM substrate (from a 10mM stock solution dissolved in DMSO) and 200μM NADPH. Samples were incubated at 30°C for 90min; this reaction time was chosen as an almost complete substrate conversion (as achieved for C12 4 after 120min) was not desirable, so as to allow comparison of the conversion values for the individual substrates and also between the two CYP4B1 isoforms.
References:
Thesseling, Florian A.;Hutter, Michael C.;Wiek, Constanze;Kowalski, John P.;Rettie, Allan E.;Girhard, Marco [Archives of Biochemistry and Biophysics,2020,vol. 679,art. no. 108216]

16424-30-9
0 suppliers
inquiry

16899-03-9
1 suppliers
inquiry