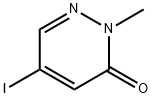
5-Iodo-2-Methylpyridazin-3(2h)-one synthesis
- Product Name:5-Iodo-2-Methylpyridazin-3(2h)-one
- CAS Number:153239-91-9
- Molecular formula:C5H5IN2O
- Molecular Weight:236.01
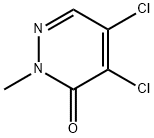
933-76-6

153239-91-9
a) General procedure for the synthesis of 5-iodo-2-methyl-2H-pyridazin-3-one (4a):[0240] 1.55 g (8.65 mmol) of 4,5-dichloro-2-methyl-2H-pyridazin-3-one was added to 50 mL of 57% aqueous hydriodic acid. The reaction mixture was heated at 137°C for 24 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was poured into a solution prepared from 20 g of sodium thiosulfate dissolved in 250 mL of water. The reaction mixture was extracted with dichloromethane and the organic phase was washed sequentially with water and saturated sodium chloride solution. After drying over anhydrous sodium sulfate, the organic phase was concentrated to dryness under reduced pressure. The resulting residue was ground with a 50:50 solvent mixture of dichloromethane-methanol and the precipitate was separated by filtration. 3.45 g of Intermediate 4a in yellow powder form was finally obtained (yield: quantitative).TLC conditions: silica gel 60F254 Merck, unfolding agent dichloromethane-ethyl acetate (70:30), Rf = 0.4.

933-76-6
132 suppliers
$7.00/250mg

153239-91-9
42 suppliers
$351.00/1g
Yield:153239-91-9 81%
Reaction Conditions:
with hydrogen iodide for 24 h;Heating;
References:
Coelho, Alberto;Sotelo, Eddy;Novoa, Héctor;Peeters, Oswald M.;Blaton, Norbert;Ravi?a, Enrique [Tetrahedron,2004,vol. 60,# 52,p. 12177 - 12189]

74-88-4
356 suppliers
$15.00/10g
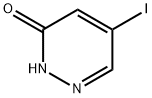
825633-94-1
135 suppliers
$9.00/100mg

153239-91-9
42 suppliers
$351.00/1g